Abstract
The prognostic significance of multidrug resistance (MDR) gene expression is controversial. We investigated whether multidrug resistance gene 1 (MDR1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) mRNA expression are associated with outcomes in acute leukemia patients. At diagnosis we examined MDR1, MRP and LRP mRNA expression in bone marrow samples from 71 acute leukemia patients (39 myeloid, 32 lymphoblastic) using nested RT-PCR. The expression of each of these genes was then expressed as a ratio in relation to β-actin gene expression, and the three genes were categorized as being either 0, 1+, 2+ or 3+. MDR1, MRP and LRP mRNA expression was detected in 23.9%, 83.1% and 45.1%, respectively. LRP mRNA expression was significantly associated with resistance to induction chemotherapy in acute leukemia patients, and in the AML proportion (p=0.02 and p=0.03, respectively). MRP and high MDR1 mRNA expression was associated with poorer 2-yr survival (p=0.049 and p=0.04, respectively). Patients expressing both MRP and LRP mRNA had poorer outcomes and had worse 2-yr survival. The present data suggest that MDR expression affects complete remission and survival rates in acute leukemia patients. Thus, determination of MDR gene expression at diagnosis appears likely to provide useful prognostic information for acute leukemia patients.
Drug resistance is a major obstacle in the successful treatment and an important cause of death in acute leukemia. Such resistance may be present before begining treatment or may develop during chemotherapy. Drug resistance that extends to structurally and functionally unrelated drugs is termed multidrug resistance (MDR) (1).
Several molecular biological mechanisms have been identified as being associated with MDR (2). P-glycoprotein (PGP), also named P-170, is a product of the multidrug resistance 1 gene (MDR1) and is an ATP-dependent pump capable of expelling drugs out of cancer cells (3, 4). PGP is a transmembrane glycoprotein conferring cross-resistance to a variety of mechanistically and structurally unrelated cytotoxic drugs, such as anthracyclines, taxanes, vinca alkaloids and epipodophyllotoxins (5). Another protein, the multidrug resistance-related protein (MRP), is structurally similar to PGP and belongs to the same transmembrane transporter superfamily (6). The substrate specificity of MRP is similar to but more limited than that of PGP, and its normal physiological role may be detoxification of intracellular oxidants (5). In addition to these two proteins, a 110 kDa protein has been identified in a PGP-negative MDR lung cancer cell line. This protein was termed the lung resistance protein (LRP) and acts as a major vault protein in humans (7). Despite the identification of these proteins, the pathways that result in drug resistance in leukemic cells remain largely uncharacterized. New information regarding drug resistance mechanisms is likely to increase the chances of cure either through development of new drugs or by means of strategies that may modulate or reverse resistance (8). While drug resistance gene expression has been studied in acute leukemia (9-12), the value of MDR1, MRP and LRP gene expression as independent predictors of treatment success is still controversial.
In the present study, we investigated whether outcomes in acute leukemia patients were associated with MDR1, MRP and LRP mRNA expression.
The study involved 71 patients (34 males and 37 females) diagnosed with acute leukemia between January 1998 and January 1999. The median age of the patients was 30 yr (range, 3 months - 75 yr). Diagnosis and classification of acute leukemia was made according to the French-American-British (FAB) criteria and immunophenotype analyses. There were 39 acute myeloid leukemia (AML) and 32 acute lymphoblastic leukemia (ALL) patients. Among 39 AML patients, eleven patients had AML M1, 11 M2, 7 M3, 6 M4, 2 M5, 1 M6 and 1 M7. The patients were classified as being good prognosis group, intermediated prognosis group and poor prognosis group according to cytogenetic results. AML patients with t(8;21), t(15;17) and inv (16) were defined as "good prognosis group", patients with 5-, 7-, 5q-, 7q-, hyperdiploidy and multiple chromosomal abnormality were defined as "poor prognosis group", and the others were defined as the "intermediate prognosis group". Among AML patients, eight patients (t(8;21), 1; t(15;17), 7) were in good prognosis group, 20 (normal karyotype, 19; t(11;19), 1) in intermediate prognosis group and 12 (trisomy 8, 2; other complex abnormality, 10) in poor prognosis group. Among ALL patients, two were in good prognosis group, 11 in intermediate prognosis group and 19 in poor prognosis group. The induction chemotherapy regimen were cytarabine plus idarubicin (AI regimen) in adult AML patients and vincristine, prednisone, daunorubicin, L-asparaginase (VPDL regimen) in adult ALL patients. Newly diagnosed childhood ALL patients were treated according to Children's Cancer Group (CCG) 1881, 1882, 1891 or 1901 protocols. Childhood AML patients were treated according to BFM (Frankfurt Munster group) 83 protocols, including cyclophosphamide and prednisone. Clinical information was obtained by reviewing charts.
Complete remission (CR) was defined as a cellularity of more than 20% with fewer than 5% blasts in the bone marrow (BM) after induction chemotherapy, and relapse as more than 5% blasts in the BM. The CR rate was calculated only using patients that underwent bone marrow analysis following initial diagnosis (64 cases).
Seventy one bone marrow aspirates collected in EDTA were obtained at the initial diagnosis. A COR-L23/R cell line was used as a positive control for MDR1 and LRP mRNA expression, and the HL60/Adr cell line for MRP mRNA expression. Negative controls included deionized RNAase-free water, and bone marrow aspirates obtained from patients with lymphoma without bone marrow involvement. β-actin mRNA amplification was used as an internal control.
Mononuclear cells were separated by density gradient centrifugation using Ficoll-Hypaque within 3 hr after bone marrow aspiration. Total cellular RNA was extracted using the RNeasy kit (Qiagen Inc., Valencia, CA, U.S.A.) according to the manufacturer's instructions.
cDNA was synthesized using the First Strand cDNA Synthesis Kit for RT-PCR (Behringer Mannheim Inc, Germany). Total RNA (1 µg) was reverse transcribed in 20 µL reaction mixture (2 µL 10X Reaction buffer [100 mM Tris, 500 mM KCL, pH 8.3 and 25 mM MgCl2], 10 mM each of four dNTPs, 1.6 µg/µL random primers, 50 U/µL RNase inhibitor and 1 µL AMV Reverse Transciptase). The reaction was performed at 70℃ for 2 min and 42℃ for 60 min. MDR1, MRP and LRP mRNA amplifications were performed after heating the reaction mixture to 99℃ for 5 min. The first round of PCR reactions involved 30 cycles of: 1 min denaturing at 94℃, 1 min annealing at 64℃ and 2 min extension at 72℃ using a thermocycler (Perkin Elmer-Cetus, Wellesley, PA, U.S.A.). The PCR reactions were carried out in a final volume of 50 µL containing 2 µg cDNA, 10 pg/µL of each primer, 0.4 U/µL taq polymerase, 200 µM of each dNTP, 5 µL 10X buffer containing 500 mM KCl, 100 mM Tris (pH 8.3) and 25 mM MgCl2. The first round of PCR was followed by a second round of 20 cycles. Amplification of β-actin mRNA (20 cycle PCR) was performed and the data obtained used to normalize any variations between samples (e.g. differences in concentration or quality of extracted RNA). PCR primer sequences are shown in Table 1. PCR products were electrophoretically separated on 2% agarose gels and visualized using ethidium bromide staining. The gel was photographed with Polaroid film (Fig. 1) and the film scanned into a computer (Sharp JX-330, Japan) for analysis. For semiquantitative measurements of mRNA, the relative ratios of MDR1, MRP or LRP mRNA expression to β-actin mRNA expression was calculated using ImageMaster software (Pharmacia, Sweden). Following the determination of this ratio, MDR genes were classified as either not expressed (0), weakly expressed (ratio 0-0.7=1+), moderately expressed (ratio 0.7-1=2+), or strongly expressed (ratio >1=3+).
Data were analyzed using chi square or Fisher exact tests. Survival curves were plotted using the Kaplan-Meier method, and differences were analyzed using log-rank tests. A p value <0.05 was deemed to indicate a significant difference. All statistical analyses were performed using SPSS version 11.5 (SPSS, Chicago, IL., U.S.A.).
We found that the proportion of acute leukemia patients expressing MDR1, MRP and LRP mRNA was 23.9%, 83.1% and 45.1%, respectively (Table 2). In terms of leukemia categories, MDR1, MRP and LRP mRNA was expressed in 23.1%, 89.4% and 48.7% respectively of AML patients, and in 25.0%, 75.0% and 40.6% respectively of ALL patients. While fewer patients expressed MDR1 mRNA compared to MRP and LRP mRNA, the rate of MDR1 mRNA expression (3+) was higher than the rate of either MRP or LRP mRNA high expression (3+) (Table 2). Univariate analysis showed no correlation between cytogenetic results and MDR1, MRP and LRP mRNA expression in AML and ALL patients.
LRP mRNA expression at initial diagnosis was associated with a lower CR rate after induction chemotherapy, with only 55.2% (16/29) of LRP-positive acute leukemia patients achieving CR, compared to 82.9% (29/35) of LRP-negative patients achieving CR (p=0.02). Similarly, for AML patients, 47.1% (8/17) of LRP-positives achieved CR, while 82.4% (14/17) of LRP-negatives achieved CR (p=0.03). MDR1 and MRP mRNA expression appeared to have statistically no significant effect on patient outcome following induction chemotherapy. However, MDR1 or MRP mRNA-positive patients showed the tendency of lower CR rates than MDR1 or MRP-negative patients (Table 3). MDR1, MRP or LRP mRNA expression had no effect on relapse rates. The multivariate logistic regression analysis of age, MDR mRNA expression and cytogenetic results indicated that the MDR mRNA expression did not give significant effects on relapse and continuous CR after induction chemotherapy.
Kaplan-Meier analysis showed that the median survival for MRP-positive acute leukemia patients was 600 days, while MRP-negative patients did not reach the median survival time. There was a significant difference in the 2-yr survival between these two groups (p=0.049) (Fig. 2). There was statistically no prognostic significance for MDR1 (p=0.05) or LRP mRNA (p=0.08) expression in acute leukemic patients in total, nor for AML or ALL patients when analyzed separately.
High level MDR1 mRNA expression was significantly associated with poorer 2-yr survival in acute leukemia patients (p=0.04) (Fig. 3), with all three such patients dying within 2 yr (20 days, 228 days and 405 days) of diagnosis. Among these patients, two were diagnosed with adult AML and one with childhood AML. Only one of these three patients achieved CR after induction chemotherapy.
While the survival time appeared to decrease with increasing MRP and LRP mRNA expression levels, this observation was not supported by statistical analysis.
Double positivity for MDR1 and MRP mRNA was detected in 17 samples (23.4%), MDR1 and LRP mRNA in 15 (21.1%) and MRP and LRP mRNA in 31 (43.7%). MDR1 and LRP positive patients also showed positivity for MRP. While 57.1% (16/28) of acute leukemia patients expressing both MRP and LRP mRNA achieved CR, 80.6% (29/36) of those not expressing both genes achieved CR (p=0.04). Expressing both MDR1 and MRP mRNA, or both MDR1 and LRP mRNA had no significant influence on the CR rate after induction chemotherapy. The median survival of MRP and LRP-double positive patients was 405 days; the other patients did not reach median survival. There was a significant difference in the 2-yr survival between the two groups (p=0.04). Expressing both MDR1 and MRP mRNA, or MDR1 and LRP mRNA had no negative impact on 2-yr survival.
The present study indicates that MDR gene expression can affect outcomes in acute leukemia patients. LRP gene expression at diagnosis appeared to be associated with resistance to induction chemotherapy in acute leukemia patients. This was not the case for MDR1 or MRP gene expression. For AML patients, while over 80% of LRP-negative subjects achieved CR, less than half of the LRP-positive subjects achieved CR (p=0.03). Therefore, LRP mRNA expression was particularly influential in outcomes following induction chemotherapy in AML patients. This correlation between LRP expression and treatment outcome is consistent with suggestions that chemosensitive leukemic cells have low LRP mRNA levels in AML (10, 13).
Interestingly, it was reported that LRP mRNA expression more strongly correlated with drug resistance than LRP protein expression (14). Sauerbrey et al. (15) suggested that LRP overexpression might be a mechanism involved in childhood ALL drug resistance. While the present data appeared to suggest LRP-positive ALL patients showed a lower probability for first remission, this observation was not supported statistically.
In AML patients, PGP/MDR1 expression was found to be an independent prognostic variable associated with reduced probability for achieving remission following conventional anthracene-containing induction regimens, and in some reports this expression was linked to inferior leukemia-free and overall survivals (16-18). However, while our data appeared to suggest that MDR1-positive AML and ALL patients had low CR rates and poor 2-yr survivals, this observation was not supported by statistical analysis. It may be that analysis of a larger number of patients would provide the statistical power for significance. The different sensitivities of the detection techniques may be responsible for the discrepant statistical significance. However, three patients with strong MDR1 mRNA expression died within 2 yr, and high levels of MDR1 mRNA were significantly associated with poor 2-yr survival in acute leukemia patients. Therefore, we need to consider that patients with high MDR1 mRNA expression may have poorer outcomes.
The reported proportion of patients positive for MDR gene expression varies widely and appears to be related to the study method employed and the patient characteristics of any particular study. In the present study, 83.1% of patients expressed MRP mRNA, and 2-yr survival in these patients was less than that in MRP-negative patients (p=0.049). Although some studies show MRP expression lacked prognostic power (19, 20), others found it was predictive of poorer outcome (21).
In the present study, MRP and LRP-double positive patients showed reduced remission rates after induction chemotherapy and poorer 2-yr overall survival compared to patients not expressing both of these genes. Only two studies have previously attempted to determine the impact of simultaneous expression of two MDR genes, and the conclusions drawn by these studies were not consistent with each other (21, 22).
The present study demonstrates that outcomes in acute leukemia patients can vary depending upon expression of MDR1, MRP and LRP mRNA. Simultaneous expression of MRP and LRP mRNA correlated with a low CR rate and poor survival. These results suggest that analysis of MDR gene expression at diagnosis of acute leukemia may provide useful prognostic information. Such data is also likely to assist in determining the mechanisms underlying drug resistance.
Figures and Tables
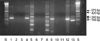 | Fig. 1RT-PCR products following MDR1, MRP and LRP mRNA amplification. Lane S, size marker; lane 1, MDR1-positive cell line (band at 243 bp); lane 2, DW; lane 3, normal bone marrow; lane 4, MDR1-positive patient; lane 5, MRP-positive line (bands at 575 and 420 bp); lane 6, DW; lane 7, normal bone marrow; lane 8, s-positive patient; lane 9, LRP-positive cell line (band at 239 bp); lane 10, DW; lane 11, normal bone marrow; lane 12, LRP-positive patient; lane 13, β-actin (internal control, band at l66 bp). (DW=deionized water (negative control)). |
References
1. Riordan JR, Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985. 28:51–75.

3. Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, Pastan I, Gottesman MM. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986. 261:7762–7770.

4. Lee NY, Bae HG, Kwon EH, Heo WB, Shin SH, Suh JS. Relation among the tests and comparison of positivity of tests for multi-drug resistance in newly diagnosed acute leukemia. Korean J Lab Med. 2003. 23:143–150.
6. Kruh GD, Chan A, Myers K, Gaughan K, Miki T, Aaronson SA. Expression complementary DNA library transfer establishes mrp as a multidrug resistance gene. Cancer Res. 1994. 54:1649–1652.
7. Scheper RJ, Broxterman HJ, Scheffer GL, Kaaijk P, Dalton WS, van Heijningen TH, van Kalken CK, Slovak ML, de Vries EG, van der Valk P. Overexpression of a M (r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistance. Cancer Res. 1993. 53:1475–1479.
8. Szabo D, Keyzer H, Kaiser HE, Molnar J. Reversal of multidrug resistance of tumor cells. Anticancer Res. 2000. 20:4261–4274.
9. Kakihara T, Tanaka A, Watanabe A, Yamamoto K, Kanto K, Kataoka S, Ogawa A, Asami K, Uchiyama M. Expression of multidrug resistance-related genes does not contribute to risk factors in newly diagnosed childhood acute lymphoblastic leukemia. Pediatr Int. 1999. 41:641–647.

10. List AF, Spier CS, Grogan TM, Johnson C, Roe DJ, Greer JP, Wolff SN, Broxterman HJ, Scheffer GL, Scheper RJ, Dalton WS. Overexpression of the major vault transporter protein lung-resistance protein predicts treatment outcome in acute myeloid leukemia. Blood. 1996. 87:2464–2469.

11. Dhooge C, De Moerloose B, Laureys G, Kint J, Ferster A, De Bacquer D, Philippe J, Benoit Y. P-glycoprotein is an independent prognostic factor predicting relapse in childhood acute lymphoblastic leukaemia: results of a 6-year prospective study. Br J Haematol. 1999. 105:676–683.

12. Gurbuxani S, Zhou D, Simonin G, Raina V, Arya LS, Sazawal S, Marie JP, Bhargava M. Expression of genes implicated in multidrug resistance in acute lymphoblastic leukemia in India. Ann Hematol. 1998. 76:195–200.

13. Xu D, Arestrom I, Virtala R, Pisa P, Peterson C, Gruber A. High levels of lung resistance related protein mRNA in leukaemic cells from patients with acute myelogenous leukaemia are associated with inferior response to chemotherapy and prior treatment with mitoxantrone. Br J Haematol. 1999. 106:627–633.

14. Laurencot CM, Scheffer GL, Scheper RJ, Shoemaker RH. Increased LRP mRNA expression is associated with the MDR phenotype in intrinsically resistant human cancer cell lines. Int J Cancer. 1997. 72:1021–1026.
15. Sauerbrey A, Voigt A, Wittig S, Hafer R, Zintl F. Messenger RNA analysis of the multidrug resistance related protein (MRP1) and the lung resistance protein (LRP) in de novo and relapsed childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2002. 43:875–879.

16. Guerci A, Merlin JL, Missoum N, Feldmann L, Marchal S, Witz F, Rose C, Guerci O. Predictive value for treatment outcome in acute myeloid leukemia of cellular daunorubicin accumulation and P-glycoprotein expression simultaneously determined by flow cytometry. Blood. 1995. 85:2147–2153.

17. Mahadevan D, List AF. Targeting the multidrug resistance-1 transporter in AML: molecular regulation and therapeutic strategies. Blood. 2004. 104:1940–1951.

18. Schaich M, Soucek S, Thiede C, Ehninger G, Illmer T. MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. Br J Haematol. 2005. 128:324–332.

19. Borg AG, Burgess R, Green LM, Scheper RJ, Yin JA. Overexpression of lung-resistance protein and increased P-glycoprotein function in acute myeloid leukaemia cells predict a poor response to chemotherapy and reduced patient survival. Br J Haematol. 1998. 103:1083–1091.

20. den Boer ML, Pieters R, Kazemier KM, Rottier MM, Zwaan CM, Kaspers GJ, Janka-Schaub G, Henze G, Creutzig U, Scheper RJ, Veerman AJ. Relationship between major vault protein/lung resistance protein, multidrug resistance-associated protein, P-glycoprotein expression, and drug resistance in childhood leukemia. Blood. 1998. 91:2092–2098.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download