Abstract
Insulin resistance is an important risk factor for coronary artery disease. However, there has been no data regarding its clinical effect on the outcomes of percutaneous coronary intervention (PCI) in non-diabetic patients. We analyzed 98 non-diabetic consecutive patients (59±11.5 yr, male:female=63:35) who underwent elective coronary angiography. The patients were divided into two groups: Group I (n=71; the value of HOMA-IR [homeostasis model assessment of insulin resistance] <2.6) and Group II (n=27; the value of HOMA-IR ≥2.6). In-hospital and 30-day major adverse cardiac events (MACE) were compared between the two groups. The concentrations of fasting insulin and triglyceride were significantly higher in Group II than in Group I. Significant correlations were observed between the value of HOMA-IR and body mass index (r=0.489, p<0.001), levels of total cholesterol (r=0.204, p=0.045), triglyceride (r=0.334, p=0.001) and apolipoprotein B (r=0.212, p=0.038). PCI was performed in 59 patients (60.2%). In-hospital and 30-day MACE were higher in Group II than Group I (2.4% vs. 27.8%, p=0.008; 2.4% vs. 27.8%, p=0.008). Multivariate analysis revealed that the value of HOMA-IR ≥2.6 was an independent predictor of MACE. Increased HOMA-IR level is an important prognostic indicator in non-diabetic patients underwent PCI.
Insulin resistance and hyperinsulinemia are associated with hypertension, impaired glucose tolerance, obesity and dyslipidemia (1-4). These factors are also closely related to the development of coronary heart disease. Hyperinsulinemia is characterized by low high density lipoprotein (HDL)-cholesterol and high low density lipoprotein (LDL)-cholesterol, which causes coronary arteriosclerosis. This reflects the rate of mortality due to cardiovascular diseases.
To date, euglycemic hyperinsulinemic clamp test has been accepted as a standard test that measures insulin resistance accurately (5). However, since euglycemic hyperinsulinemic clamp test is very complex as well as requires skillful technicians and many instrument, it has not been widely used in a clinical setting. Alternately, homeostasis model assessment (HOMA) index was designed to assess insulin resistance in many patients, in which the concentrations of fasting insulin and glucose are simply measured, and recently has been widelyused (6, 7).
Considering that insulin resistance is the risk factor for coronaryheart disease, it may be closely related to the prognosis of patients who underwent percutaneous coronary intervention (PCI). To date, however, few studies have been conducted to examine whether insulin resistance is correlated with the prognosis of patients who underwent PCI. Some studies have reported that severe coronary angiographic findings and stent restenosis were closely associated with insulin resistance (8, 9).
Based on the assumption that insulin resistance is closely associated with the complications after PCI or the prognosis after PCI, we conducted a 30-day follow-up clinical study to observe major adverse cardiac events (MACE) in patients who underwent PCI.
In this study, we examined 98 consecutive patients with chest pain who underwent diagnostic coronary angiography between May and September 2004. We excluded patients who were treated for diabetes mellitus; those who were newly diagnosed as diabetes mellitus; those who had thyroid or adrenal insufficiency; those who underwent PCI for coronary heart disease within a recent 6-month period; and those who underwent primary PCI for acute myocardial infarction. The clinical diagnosis on admission was stable angina pectoris in 29.6% (29 patients) and unstable angina pectoris in 70.4% (69 patients). On admission, patients were interviewed to collect such data as the risk factors for coronary heart disease, the personal history of smoking, diabetes mellitus and hypertension, the family history and the past history of myocardial infarction or stroke. All patients gave informed consent according to a protocol approved by the Chonman National University Hospital Ethics Committee.
In our patients, samples were collected from venous blood after overnight fasting, and blood chemistry was performed. Fasting plasma glucose and insulin were measured. Then, the following parameters were measured: 1) the concentrations of serum lipid including total cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol, Apolipoprotein AI (Apo AI), Apolipoprotein B (Apo B) and lipoprotein (a) [Lp (a)]; 2) leukocyte count, monocyte count, erythrocyte sedimentation rate (ESR) and high-sensitivity C-reactive protein (hsCRP); and 3) the concentrations of fibrinogen, fibrinogen degradation product (FDP) and homocysteine.
Insulin resistance was calculated by the homeostasis model assessment of insulin resistance (HOMA-IR), proposed by Mattews et al., whose formula was: HOMA-IR (mg/dL×U/mL)=fasting glucose (mg/dL)×fasting insulin (U/mL)/405 (6).
To determine cut-off points of the HOMA-IR as predictors of in-hospital and 30-day adverse cardiac events, receiver operating characteristics (ROC) analyses were performed. The area under curve (AUC) of HOMA-IR was 0.738 (p=0.006). Cut-off points of HOMA-IR for in-hospital and 30-day cardiac events, determined by ROC analysis, were 2.56, 2.60, respectively. Therefore, we used 2.6 as a cut-off point for the analysis in clinical usefulness. The cut-off point for in-hospital and 30-day adverse cardiac events yielded sensitivities of 66.8% and 77.9% and specificities of 68.2% and 70.2%, respectively.Based on the cut-off value of 2.6, therefore, we divided patients into Group I (the value of HOMA-IR <2.6) and Group II (the value of HOMA-IR ≥2.6).
We accessed left or right femoral artery by the Seldinger method or accessed radial artery, and performed coronary angiography using the Judkins method. Coronary angiographic findings were interpreted by two examiners who were blinded to patients' profile of insulin resistance. Significant stenosis was defined as a luminal narrowing of 50% or greater (10).
PCI was performed according to current clinical practice at physician's discretion. Stenting was performed in cases whom percutaneous balloon dilatation produced suboptimal results that the residual stenosis was more than 30%, and the dissection was developed (11, 12). On diagnostic coronary angiography,the patency of the treated artery was evaluated by the Thrombolysis In Myocardial Infarction (TIMI) score. Successful reperfusion following PCI was defined as TIMI III flow with <25% residual stenosis (13).
In all patients, aspirin (300 mg/day) and clopidogrel (150 mg/day) were loaded or aspirin (100 mg daily) and clopidogrel (75 mg daily) started >3 days before procedure. An intravenous bolus of 5,000 U of unfractionated heparin was given, and then additional heparin boluses were given to maintain activated clotting time >300 sec during procedure. Aspirin (100 mg/day) and clopidogrel (75 mg/daily for 30 days) were prescribed to all patients after procedure.
In-hospital adverse outcomes were defined as the overall procedure was not successfully done since coronary wire or balloon catheter could not pass through the lesion; myocardial infarction was developed following PCI; patients underwent repeated PCI or emergent coronary artery bypass graft (CABG) surgery for target vessel revascularization (TVR). Myocardial infarction was defined as a CK-MB elevation greater than three times the upper normal limit or a new change of EKG findings. Between Group I and II, we compared MACE such as death, myocardial infarction and target vessel revascularization during a 30-day period.
Statistical analysis was done using SPSS® Ver.12.0 for Windows. All data were represented as mean±standard deviation. Intergroup analysis was done using independent t-test and χ2 test, and intragroup analysis was done using paired t-test. Multivariate analysis was done to determine the factors related to MACE. Spearman correlation was used to examine the relationship between HOMA-IR and other laboratory findings. Statistical significance was set at p<0.05.
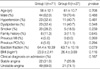
In studied patients, the mean age was 59.8±11.5 yr and a male-to-female ratio was 1.8:1. The mean value of HOMA-IR was 2.3±1.69, and 71 patients were into Group I (the value of HOMA-IR<2.6) and 27 patients into Group II (the value of HOMA-IR≥2.6). Between Group I and II, there were no significant differences in cardiovascular risk factors including hypertension, diabetes mellitus and smoking (Table 1).
The concentrations of fasting plasma glucose were 101.2±13.89 mg/dL in Group I and 108.4±9.79 mg/dL in Group II, and those of fasting insulin were 11.9±4.99 µU/mL in Group I and 31.8±14.23 µU/mL in Group II (Table 2). The concentration of triglyceride was significantly higher in Group II than in Group I (151.4±192.43 mg/dL vs. 94.5±62.86 mg/dL, p=0.028). HOMA-IR was correlated with body mass index (r=0.489, p<0.001), levels of total cholesterol (r=0.204, p=0.045), triglyceride (r=0.334, p=0.001), and apolipoprotein B (r=0.212, p=0.038).
Coronary angiography revealed the presence of significant stenosis in 64 patients (Group I; 43 patients, Group II; 21 patients). There was no significant differences in the prevalence of multi-vessel disease between the two groups (p=0.164). The lesion locations were left anterior descending artery (LAD) in 72.1%, left circumflex artery (LCX) in 16.3% and right coronary artery (RCA) in 11.6% of Group I; and LAD in 47.6%, LCX in 19.0% and RCA in 33.3% of Group II (p=0.085). According to the American College of Cardiology and American Heart Association (ACC/AHA) classification, Type B1 was demonstrated in 44.2%, Type B2 in 34.9% and Type C in 20.9% of Group I; and Type B1 in 28.6%, Type B2 in 33.3% and Type C in 33.3% of Group II (p=0.283). Preprocedural TIMI flow grades were grade 0 in 16.3%, grade I in 7.0%, grade II in 9.3% and grade III in 67.4% of Group I; and grade 0 in 19.0%, grade I in 9.5%, grade II in 19.0% and grade III in 52.5% of Group II (p=0.623).
In 59 patients, PCI was performed with an angiographic success rate of 94.9% (Table 3). The procedural failure due to crossing failure of guide wire or balloon catheter occurred in no patient (0.0%) of Group I and 2 patients (11.1%) of Group II. Procedure-related complications included one case of myocardial infarction (2.4%) in Group I; and two cases of myocardial infarction (11.1%), one case of emergent CABG (5.6%) in Group II. The procedure-related complications were more prevalent in Group II than Group I (p=0.008). The MACE at 30-day follow-up was 1 case (2.4%) of TVR in Group I and 4 cases (22.2%) of TVR in Group II, and the incidence of MACE was significantly higher in Group I than Group II (p=0.008).
Multivariate analysis revealed that the value of HOMA-IR ≥2.6 was the independent prognostic indicators for MACE (p=0.048) (Table 4).
Our clinical study indicated that high HOMA-IR value is related with the high incidence of complications and shortterm major adverse cardiac events (MACE) following PCI even in non-diabetic patients.
Insulin resistance plays a crucial role in the development of metabolic disorder accompanied by obesity, dyslipidemia, diabetes mellitus, impaired glucose tolerance and hypertension; and is also closely related to the incidence of cardiovascular disease (14-22). Hyperinsulinemia is an only indirect indicatorfor insulin sensitivity. Several large-scale studies have revealed that hyperinsulinemia is closely associated with the mortality due to cardiovascular disease (14, 21, 23). To date, however, few large-scale studies have been conducted to examinethe relationship between insulin resistance and coronary heart disease (24, 25). Recently, IRAS (insulin resistance atheroscleorsis study) group has conducted large-scale epidemiologic studies, and has reported that insulin resistance rather than insulin concentration is an independent, powerful risk factor for coronary heart disease (26).
The mechanism by which insulin resistance provokes cardiovascular disease is mainly associated with the development of metabolic syndrome. It is well established that non-diabetic patients with insulin resistance exhibits high concentration of serum triglyceride, hypertension and low concentration of HDL-cholesterol (1, 4, 23, 24). In recent years, among several types of apolipoprotein that determines the conformational stability and the metabolic direction of lipoprotein, apolipop-rotein B has been elevated particularly in patients with insulin resistance (27, 28). This has indicated that apolipoprotein B is associated with the development of cardiovascular disease. In addition, insulin resistance has been reported to reduce flow-mediated vasodilation (FMD) of brachial artery, and to thereby trigger the endothelial dysfunction (20). The present study has shown that the value of HOMA-IR was correlated with levels of triglyceride and inversely correlated with that of HDL-cholesterol, which is similar to the results of previous studies (1, 23, 24).
To date, only few studies based on coronary angiography have examined the relationship between insulin resistance and coronary atherosclerosis (8, 24, 25). According to Takezako et al., the profile of insulin resistance based on HOMA-IR model was correlated with severity of coronary atherosclerosis based on Gensini's score (8). Other studies have reported that the incidence of MACE following PCI was higher in non-diabetic patients with high concentration of HbA1c than those with low concentration of HbA1c, although these studies did not evaluate using insulin resistance (29). Here, the concentration of HbA1c was lower in non-diabetic patients than in diabetic patients although it was 'high'. On the other hand, some studies have reported that hyperinsulinemia and insulin resistance measured by HOMA are closely associated with restenosis following stenting in non-diabetic patients (9, 30). In our series, we have predicted that since insulin resistance is the risk factor for coronary atherosclerosis and is associated with the mortality due to cardiovascular disease, it will affect the prognosis following PCI. As predicted, the results were that the higher value of HOMA-IR was associated with the higher incidence of MACE, since more TVR was performed in cases with higher value of HOMA-IR. Although our results were based on non-diabetic patients, these findings suggested that patients with high insulin resistance exhibited high incidences of complex and heavy calcified lesions, commonly noted in coronary artery of patients with diabetes mellitus. Henceforth, comparative studies will be conducted regarding this matter between diabetic and non-diabetic patients.
The limitation of present study has disclosed that it examined the small number of patients within a short period of time. To date, however, few studies have been conducted to examine whether insulin resistance is correlated with the prognosis following PCI. For this reason, the present study has its own value that it is a preliminary study for further large-scale studies. Moreover, the present study failed to testify the reproducibility since it did not measure the value of HOMA-IR in a repetitive manner during the period of admission. The concentrations of serum glucose and insulin can be altered at each different measuring time, although the present study measured them only once in the morning that diagnostic coronary angiogram was performed. However, HOMA-IR index is well reflected in euglycemic hyperinsulinemic clamp test, a standard test, and the clinical usefulness has been well established.
In conclusion, insulin resistance is associated with poor prognosis in non-diabetic patients after PCI. However, long-term large clinical follow-up studies should be conducted in diabetic and non-diabetic patients.
Figures and Tables
Table 1
Baseline clinical characteristics of Group I (the value of HOMA-IR [homeostasis model assessment of insulin resistance] <2.6) and Group II (the value of HOMA-IR ≥2.6)
References
1. Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, tri-glyceride and insulin concentrations. J Intern Med. 1992. 231:25–30.

3. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Task-inen MR, Groop L. Cardiovascular morbidity and mortality associ-ated with the metabolic syndrome. Diabetes Care. 2001. 24:683–689.

4. Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resis-tance in nondiabetic subjects. Diabetes. 1998. 47:793–800.
5. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979. 237:E214–E223.

6. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.

7. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio heart study. Diabetes Care. 1997. 20:1087–1092.

8. Takezako T, Saku K, Zhang B, Shirai K, Arakawa K. Insulin resistance and angiographical characteristics of coronary atherosclerosis. Jpn Circ J. 1999. 63:666–676.

9. Radke PW, Voswinkel M, Reith M, Kaiser A, Haager PK, Hanrath P, Hoffmann R. Relation of fasting insulin plasma levels to restenosis after elective coronary stent implantation in patients without diabetes mellitus. Am J Cardiol. 2004. 93:639–641.

10. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Circulation. 1975. 51:5–40.
11. Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990. 82:1193–1202.

12. Moussa I, Di Mario C, Reimers B, Akiyama T, Tobis J, Colombo A. Subacute stent thrombosis in the era of intravascular ultrasound-guided coronary stenting without anticoagulation: frequency, predictors and clinical outcome. J Am Coll Cardiol. 1997. 29:6–12.

13. Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996. 93:879–888.
14. Pyorala M, Miettinen H, Laakso M, Pyorala K. Hyperinsulinemia and the risk of stroke in healthy middle-aged men: The 22-year follow-up results of the Helsinki Policemen Study. Stroke. 1998. 29:1860–1866.
15. Sung KC, Kim BJ, Kim BS, Kang JH, Lee MH, Park JR, Rhee EJ, Lee WY, Kim SW, Kim H, Lee KB, Ryu SH. In normoglycemic Koreans, insulin resistance and adipocity are independently correlated with high blood pressure. Circ J. 2004. 68:898–902.

16. Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994. 25:66–73.

17. Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV, Saad MF, Savage P, Bergman R. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Insulin sensitivity and atherosclerosis. Circulation. 1996. 93:1809–1817.

18. Haffner SM, D'Agostino R, Mykkanen L, Hales CN, Savage PJ, Bergman RN, O'Leary D, Rewers M, Selby J, Tracy R, Saad MF. Proinsulin and insulin concentrations in relation to carotid wall thickness: Insulin Resistance Atherosclerosis Study. Stroke. 1998. 29:1498–1503.
19. Bavenholm P, Proudler A, Tornvall P, Godsland I, Landou C, de Faire U, Hamsten A. Insulin, intact and split proinsulin, and coronary artery disease in young men. Circulation. 1995. 92:1422–1429.

20. Mizuno T, Matsui H, Imamura A, Numaguchi Y, Sakai K, Murohara T, Okumura K. Insulin resistance increases circulating malondialdehyde-modified LDL and impairs endothelial function in healthy young men. Int J Cardiol. 2004. 97:455–461.

21. Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996. 334:952–957.

22. Yanase M, Takatsu F, Tagawa T, Kato T, Arai K, Koyasu M, Horibe H, Nomoto S, Takemoto K, Shimizu S, Watarai M. Insulin resistance and fasting hyperinsulinemia are risk factors for new cardiovascular events in patients with prior coronary artery disease and normal glucose tolerance. Circ J. 2004. 68:47–52.

23. Yarnell JW, Sweetnam PM, Marks V, Teale JD, Bolton CH. Insulin in ischemic heart disease: are associations explained by triglyceride concentrations: the Caerpholly prospective study. Br Heart J. 1994. 171:293–296.
24. Young MH, Jeng CY, Sheu WH, Shieh SM, Fuh MM, Chen YD, Reaven GM. Insulin resistance, glucose intolerance, hyperinsulinemia and dyslipidemia in patients with angiographycally demonstrated coronary artery disease. Am J Cardiol. 1993. 72:458–460.
25. Shinozaki K, Suzuki M, Ikebuchi M, Hara Y, Harano Y. Demonstration of insulin resistance in coronary artery disease documented with angiography. Diabetes Care. 1996. 19:1–7.

26. Rewers M, Zaccaro D, D'Agostino R, Haffner S, Saad MF, Selby JV, Bergman R, Savage P. Insulin Resistance Atherosclerosis Study Investigators. Insulin sensitivity, insulinemia, and coronary artery disease: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004. 27:781–787.

27. Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic artery very low density lipoprotein overproduction in insulin resistance: evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem. 2000. 275:8416–8425.
28. Hwang ST, Sung KC, Kim BJ, Kim BS, Kang JH, Lee MH, Park JR, Rhee EJ, Lee WY, Kim SW, Jeon WK, Lee SJ. Insulin resistance and apolipoprotein B as a metabolic syndrome risk factor in normal glucose tolerance. Korean J Med. 2004. 66:156–166.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download