Abstract
Our aim was to demonstrate the potential of first-trimester embryofetoscopy for prenatal diagnosis in a continuing pregnancy. A patient at risk for giving birth to an infant with short rib-polydactyly syndrome, type II (Majewski), presented for prenatal diagnosis at 9 weeks of gestation. A 1 mm semirigid fiberoptic endoscope with an 18 gauge examination sheath and a single-chip digital camera were used for transabdominal embryofetoscopy. Transabdominal embryofetoscopy was performed at 13 weeks of gestation. Direct visualization of the fetus was achieved and no gross limb or facial abnormalities were seen. This case shows that embryofetoscopy is a useful tool for early diagnosis in high-risk patients in the first trimester for continuing pregnancies.
Early prenatal diagnosis of congenital anomalies often approaches the limits of ultrasonography in the first trimester of pregnancy. Further evaluation can be performed by embryofetoscopy which provides direct visualization of the embryo and fetus.
With the development of small fiberoptic endoscopes, transabdominal embryofetoscopy has been introduced in the first trimester of continuing pregnancies for early prenatal diagnosis of genetic syndromes with recognizable external fetal abnormalities that are beyond the resolution of current ultrasound in the first trimester (Table 1) (1-7).
This report of a case demonstrates the potential of first trimester embryofetoscopy, used in a patient whose fetus was at high risk for short rib-polydactyly syndrome (SRPS), type II (Majewski).
A 30-yr-old married woman, gravida 4, termination of pregnancy 3, presented for prenatal diagnosis at 9 weeks of gestation in July, 2003 because she had had three babies affected by SRPS resulting in termination of each pregnancy consecutively since 1997.
Her first pregnancy, at the age of 24, was terminated at 23 weeks of gestation at local private clinic due to oligohydramnios and short limbs of the fetus. She was also told that the baby had cleft lip and polydactyly on gross examination without an autopsy. In the following year, she was referred to our hospital at 26 weeks of gestation with suspicious short limbs by ultrasound. SRPS, type II (Majewski type) was diagnosed after her second termination by the presence of narrow constricted thorax and short ribs, median cleft lip and palate, pre- and postaxial polysyndactyly in both hands and feet, renal tubular and glomerular cysts and short long bones including tibia. She was informed that recurrence risk of having another affected baby is 25% since SRPS is an autosomal recessive disorder. She became pregnant again at the age of 26 and prenatal sonographic evaluation was begun in our unit at 11+5 weeks of gestation. The short limbs suggested by conventional ultrasound at 18+2 weeks and preaxial polydactyly of hand and foot by 3D ultrasound at 21 weeks of gestation made her third pregnancy terminated. A cleft lip was also noticed.
From the first beginning of prenatal care for her current pregnancy, early embryofetoscopic evaluation was suggested for the prenatal diagnosis of the SRPS because an embryofetoscope has been available in our unit since 2001. A transvaginal scan revealed that a crown-rump length (2.55 cm) of the fetus was compatible with clinical dates (9+3 weeks). Combined screening test for chromosomal abnormalities with fetal nuchal translucency (1.2 mm) and maternal serum biochemistry of free β-hCG (1.02 MoM) and PAPP-A (0.53 MoM) was negative (<1:300 ) at 11 weeks of gestation. Fetal digits and toes could not be delineated. At 12+4 weeks of gestation, a transabdominal embryofetoscopy was offered and she was informed about the procedure, the risks and even the possibility of impaired visualization. The following week, the couple elected to undergo an embryofetoscopy. Informed consent was obtained. An ultrasonography was performed for localization of the fetus and the placenta: a live fetus was in a supine position with the breech presentation and normal biometry of 6.99 cm CRL (13+2 weeks), 2.64 cm BPD (14+5 weeks), 8.96 cm HC (14+1 weeks), 7.43 cm AC (14+0 weeks), and 0.73 cm FL (12+2 weeks). The placenta was located anteriorly (Fig. 1).
Embryofetoscopy was performed in the following manner. The abdomen was cleansed with a betadine solution. Under local anesthesia with 1% lidocaine hydrochloride solution given into the subcutaneous tissues down to the myometrium, an 18-gauge needle (1.3 mm diameter) was inserted transabdominally with ultrasound guidance through the uterine wall and the lower margin of anterior placenta into the amniotic cavity. The 1 mm endoscope (Karl Storz, Tuttlingen, Germany), connected to a xenon light source, was placed through the lumen of a 18-gauge needle after removal of the stylet and systematic visualization of the fetus was begun with gentle movements of the needle.
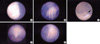
The face, eyes, nose and lips (Fig. 2A) appeared to be normal. The hands were clearly seen and there was no polydactyly or syndactyly of the fingers (Fig. 2B, C). The fetal cord insertion site was clearly seen. The external genitalia was seen and still somewhat indeterminate but suggestive of a male (Fig. 2D). Both feet were seen and again, there was no poly- or syndactyly of the toes (Fig. 2E). Direct visualization of the fetus was achieved and no gross limb or facial abnormalities were seen. The duration of the procedure was 15 min. She was discharged 2 days after procedure without any complications of fluid leakage, bleeding or uterine contraction. Follow-up ultrasound in the second trimester was advised and was performed so that normal biometry could be obtained. At 20+4 weeks of gestation, normal fetal anatomy and adequate femur length were confirmed. Serial ultrasound examinations were performed throughout the remainder of pregnancy and continued to reveal normal fetal growth and development without evidence of fetal malformations.
A normal full-term female infant was born at 39+3 weeks of gestation via a normal vaginal delivery. The infant weighed 3,250 g and was 20 inches in length. On physical examination, there was no evidence of facial or limb abnormalities. At our last follow-up at 2-months of age, the infant weighed 6,427 g and continued to do well with a normal ophthalmologic examination.
Short rib-polydactyly syndrome is a lethal skeletal dysplasia with marked limb reduction, narrow constricted thorax, short horizontal ribs, pre- and postaxial polydactyly and frequent cardiac defects and cystic renal dysplasia. The Majewski type (type II) has additional cleft lip/plalate and disproportionately shortened tibia (8-10).
SRPS is an autosomal recessive disorder with 25% recurrence risk (11). Considering the limitations of therapy, prenatal diagnosis with selective termination of pregnancy is an important option for couples at risk. At the present time, the responsible gene for disorder has not been identified and therefore, the only method of prenatal diagnosis has to be made on the detection of its phenotypic manifestations.
The prenatal diagnosis of Majewski type has been made in fetuses at risk by identification of short tibia, polydactyly, and cleft lip at fetoscopy using 1.7 mm diameter of endoscope at 16 weeks of gestation (12), or severe micromelia, short ribs with narrow thorax, and polydactyly at ultrasound (13, 14). The earliest sonographic diagnosis has been made at the 16th week of gestation (14). Despite the use of vaginal ultrasound, fetal cleft lip has not been diagnosed until 14 weeks of gestation (15, 16) and is usually recognized during the second trimester.
The fingers and toes of the fetus could always be clearly identified with embryoscopy as early as 9 weeks of gestation (17). Prenatal diagnosis of cleft lip was made at 11 weeks of gestation using embryoscopy (18). Therefore, embryofetoscopy offers a distinct advantage over current diagnostic techniques in that it can assess limb while also ruling out facial anomalies early in gestation.
Recently first-trimester embryofetoscopy has been utilized for early prenatal diagnosis of external developmental defects to either confirm or rule out. Our use of thin gauge needle embrofetoscopy in a pregnancy at risk for SRPS highlights the potential of this technique for investigating early in gestation the anatomical features of the fetus and embryo in utero.
Care should be taken in determining a fetal sex by external genitalia in the first trimester. Until 11 weeks of gestation the external genitalia of the two sexes are similar in appearance; the phallus is as big in the female as in the male and develops into penis or clitoris at 11 weeks of gestation. Labium minora and majora are formed from 11 to 14 weeks of gestation (19). Our embryofetoscopy at 13 weeks of gestation demonstrated the fetus looked male. We would not see the genitalia during the follow up fetal ultrasound examination after embryofetoscopy. But female external genitalia was observed after birth. During first-trimester embryofetoscopy it is pointed out again that distinguishing features of the external genitalia appear in 11 weeks of gestation but the external genitalial organs are not fully differentiated into male or female until 14 weeks of gestation (20). Occasional sex reversal (46, XY with female phenotype) can occur in type I and ambiguous genitalia can be seen in type II (10). Chromosomal study of the peripheral blood after birth confirmed a normal female, 46, XX although SRPS had been excluded.
Published data do not exist regarding morbidity specifically related to transabdominal embryofetoscopy in continuing pregnancies. The risks of fetal loss, infection, and amniotic membrane rupture are expected to be similar to those associated with early amniocentesis (21, 22). This procedure has a fetal loss rate of 2-2.5% (23, 24). The post procedure courses of the patient in this report were completely uneventful. There had been no evidence of damage to the retina in infants born after first trimester transcervical embryoscopy (25).
This report demonstrates the confirmatory potential of transabdominal embryofetoscopy in prenatal diagnosis. It is emphasized that the risks of first-trimester embryofetoscopy still remain to be established.
Figures and Tables
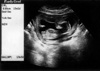 | Fig. 1Transabdominal sonogram of the fetus in supine position with the breech presentation and a crown-rump length of 6.99 cm and the anterior placenta before embryofetoscopy. |
ACKNOWLEDGEMENTS
We would like to thank Professor E. Albert Reece, Temple University School of Medicine, Philadelphia, U.S.A. for his valuable advice on the setting up the embryofetoscopy unit.
References
1. Quintero RA, Abuhamad A, Hobbins JC, Mahoney MJ. Transabdominal thin gauge embryo-fetoscopy: a technique for early prenatal diagnosis and its use in the diagnosis of a case of Meckel Gruber syndrome. Am J Obstet Gynecol. 1993. 168:1552–1557.
2. Ginsberg NA, Zbarz D, Strom C. Transabdominal embryoscopy for the detection of Carpenter syndrome during the first trimester. J Assist Reprod Genet. 1994. 11:373–375.

3. Hobbins JC, Jones OW, Gottesfeld S, Persutte W. Transvaginal ultrasonography and transabdominal embryoscopy in the first-trimester diagnosis of Smith-Lemli-Opitz syndrome, type II. Am J Obstet Gynecol. 1994. 171:546–549.

4. Reece EA, Homko CJ, Wiznitzer A, Goldstein I. Needle embryofetoscopy and early prenatal diagnosis. Fetal Diagn Ther. 1995. 10:81–82.

5. Reece EA, Homko CJ, Koch S, Chan L. First-trimester needle embryofetoscopy and prenatal diagnosis. Fetal Diagn Ther. 1997. 12:136–139.

6. Ville Y, Berbard JP, Doumerc S, Multon O, Fernandez H, Frydman R, Barki G. Transabdominal fetoscopy in fetal anomalies diagnosed by ultrasound in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 1996. 8:11–15.

7. Ville Y, Khalil A, Homphray T, Moscoso G. Diagnostic embryoscopy and fetoscopy in the first trimester of pregnancy. Prenat Diagn. 1997. 17:1237–1246.

8. Majewski F, Pfeiffer RA, Lenz W, Muller R, Feil G, Seiler R. Polysyndaktylie, verkurzte Gliedmassen und Genitalfehlbildungen: Kennzeichen eines selbstandigen Syndroms? Z Kinderheilkd. 1971. 111:118–138.
9. Spranger J, Grimm B, Weller M, Weissenbacher G, Herrmann J, Gilbert E, Kreplër R. Short Rib-Polydactyly (SRP) syndromes, Types Majewski and Saldinö-Noonan. Z Kinderheilkd. 1974. 116:73–94.
10. Jones KL. Short rib polydactyly syndrome. Smith's recognizable patterns of human malformation. 1997. 5th ed. Philadelphia: WB Saunders;334–337.
11. Wu MH, Kuo PL, Lin SJ. Prenatal diagnosis of recurrence of short rib poly-dactyly syndrome. Am J Med Genet. 1995. 55:279–284.
12. Toftager-Larsen K, Benzie RJ. Fetoscopy in prenatal diagnosis of the Majewski and the Saldino-Noonan types of the short rib-polydactyly syndromes. Clin Genet. 1984. 26:56–60.

13. Thomson GS, Reynolds CP, Cruickshank J. Antenatal detection of recurrence of Majewski dwarf (short rib-polydactyly syndrome type II Majewski). Clin Radiol. 1982. 33:509–517.

14. Gembruch U, Hansmann M, Fodisch HJ. Early prenatal diagnosis of short rib-polydactyly (SRP) syndrome type I (Majewski) by ultrasound in a case at risk. Prenat Diagn. 1985. 5:357–362.

15. Bronshtein M, Mashiah N, Blumenfeld I, Blumenfeld Z. Pseudo-prognathism: an auxiliary ultrasonographic sign for transvaginal ultrasonographic diagnosis of cleft lip and palate in the early second trimester. Am J Obstet Gynecol. 1991. 165:1314–1316.
16. Bronshtein M, Gershoni-Baruch R. Prenatal transvaginal diagnosis of the ectro-dactyly, ectodermal dysplasia, cleft palate (EEC) syndrome. Prenat Diagn. 1993. 13:519–522.

17. Dumez Y, Mandelbrot L, Dommergues M. Diagnostic embryoscopy, 50 cases of early first trimester prenatal diagnosis. Prenat Diagn. 1992. 12:S10.
18. Dommergues M, Lemerrer M, Couly G, Delezoide AL, Dumez Y. Prenatal diagnosis of cleft lip at 11 menstrual weeks using embryoscopy in the van der Woude syndrome. Prenat Diagn. 1995. 15:378–381.

19. England MA. Genitals: external. A colour Atlas of Life before Birth, Normal fetal development. 1990. London: Wolfe Medical Publications Ltd;157–162.
20. Moore KL. Development of the external genitalia. The Developing Human. Clinically Oriented Embryology. 1988. 4th ed. Philadelphia: Saunders;271–273.
21. Henry GP, Miller WA. Early amniocentesis. J Reprod Med. 1992. 37:396–402.
22. Hanson FW, Zorn EM, Tennant FR, Marianos S, Samuels S. Amniocentesis before 15 weeks gestation: Outcome, risks and technical problems. Am J Obstet Gynecol. 1987. 156:1524–1531.

23. Penso CA, Sandstrom MM, Garber MF, Ladoulis M, Stryker JM, Benacerraf BB. Early amniocentesis: report of 407 cases with neonatal follow-up. Obstet Gynecol. 1990. 76:1032–1036.
24. Nicolaides K, Brizot M, Patel F, Snijders R. Comparison of chorionic villous sampling and amniocentesis for fetal karyotyping at 10-13 weeks' gestation. Lancet. 1994. 334:435–439.
25. Dumez Y, Daffos F. Guillet P, editor. L'echographie interventionelle en medecine foetale. Echo-graphie des malformations foetales. 1990. Paris: Vigot;407–408.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download