Abstract
Recently, it was reported that fenestration of the lamina terminalis (LT) may reduce the incidence of shunt-dependent hydrocephalus in aneurysmal subarachnoid hemorrhage (SAH). The authors investigated the efficacy of the LT opening on the incidence of shunt-dependent hydrocephalus in the ruptured anterior communicating artery (ACoA) aneurysms. The data of 71-ruptured ACoA aneurysm patients who underwent aneurysmal clipping in acute stage were reviewed retrospectively. Group I (n=36) included the patients with microsurgical fenestration of LT during surgery, Group II (n=35) consisted of patients in whom fenestration of LT was not feasible. The rate of shunt-dependent hydrocephalus was compared between two groups by logistic regression to control for confounding factors. Ventriculo-peritoneal shunts were performed after aneurysmal obliteration in 18 patients (25.4%). The conversion rates from acute hydrocephalus on admission to chronic hydrocephalus in each group were 29.6% (Group I) and 58.8% (Group II), respectively. However, there was no significant correlation between the microsurgical fenestration and the rate of occurrence of shunt-dependent hydrocephalus (p>0.05). Surgeons should carefully decide the concomitant use of LT fenestration during surgery for the ruptured ACoA aneurysms because of the microsurgical fenestration of LT can play a negative role in reducing the incidence of chronic hydrocephalus.
Frequency of hydrocephalus after surgical operation about aneurysmal subarachnoid hemorrhage (SAH) is reported variously as 6-67% and the occurrence time is also varied from several days to years (1-4). Hydrocephalus is classified into the acute form (0-3 days after hemorrhage), subacute form (4-13 days after hemorrhage), and chronic form (over the 14 days after hemorrhage) according to the occurrence time (5). It is thought that acute hydrocephalus occurred by early compromise of cerebrospinal fluid (CSF) circulation and malabsorption due to the blood clot in the ventricular system, especially the fourth ventricle, and subsequent fibrosis and adhesion as a leptomeningeal reaction to the blood clot in the subarachnoid space have been contributing to the development of chronic hydrocephalus (4, 6-9). Chronic hydrocephalus, which has been indolent for more than 2 weeks, is needed for the shunt operation in almost all cases (5), and that occurred in about 20% of SAH patients (10-13).
Age of the patient, the mental status on admission, the location of ruptured aneurysms, the presence of acute hydrocephalus, and also intraventricular hemorrhage have been proposed as significant factors for the development of chronic hydrocephalus after operations for cerebral aneurysm (14-16). Recently there are some reports that lamina terminalis (LT) fenestration during aneurysm surgery by the pterional approach may decrease the incidence of the shunt-dependent hydrocephalus (17, 18). Komotar et al. reported the retrospectively analyzed results of 582 cerebral aneurysm patients, in which fenestration of LT reduced the overall shunt rate and the rate of conversion from acute hydrocephalus on admission to shunt-dependent hydrocephalus by more than 80% (19).
This study aimed to investigate the effect of LT fenestration among the several factors that influence the incidence of hydrocephalus upon the shunt rate of hydrocephalus in anterior communicating artery (ACoA) aneurysm patients, and suggest guidelines for the management of hydrocephalus after aneurysmal surgery.
Data from the clinical records of 71 patients with SAH by ACoA aneurysmal rupture, who were operated on by aneurysmal clipping through a standard pterional approach in the acute stage of hemorrhage (within 3 days after rupture) were selected between January 2000 and December 2003. Two patients who died within 2 weeks after surgical neck clipping were excluded in this study.
All patients confirmed to have ACoA aneurysmal ruptures were invariably surgically treated through a standard pterional approach and any extraventricular or lumbar drainage was not performed before craniotomy. Brain swelling was managed principally by the opening and drainage of cistern. However, intraoperative ventricular diversion (ventriculostomy) was performed only when the severe brain swelling could not be controlled by mannitol or anesthetic techniques. Blood clots in the cisterns were irrigated and sucked out meticulously after surgical clipping of the aneurysm. A self-retaining retractor was adjusted parallel to the ACoA to fully expose the LT that could be observed as a bluish, usually bulging membrane, in a medial position behind the optic chiasm. An incision was made strictly in the lower midline in the LT, to avoid damage to the vascular structures around the optic apparatus, and cerebrospinal fluid (CSF) was drained freely through the aperture. All the patients were treated conservatively for maintaining the proper hemodynamic status by use of ordinary calcium channel blocker and hypervolemic therapy during the postoperative period.
Indications for ventriculo-peritoneal (V-P) shunts were decided by radiological and clinical criteria. Radiographic hydrocephalus was defined as computed tomography (CT) imaging which demonstrated the evidence of increased ventricular size associated with the following findings: rounded aspect of the frontal horns, enlargement of the third ventricle and temporal horns, a diminished cortical sulcal pattern (sulci and gyral effacement), and the presence of periventricular lucency. Clinical criteria were neurological deterioration including mental status deterioration, gait disturbance, urinary incontinence, and memory disturbance. V-P shunt for hydrocephalus was carried out as an ordinary method.
All informations were obtained from medical records and analyzed retrospectively. The data for all 71 patients were analyzed for the following factors at admission: patient's age, sex, Hunt-Hess grade, Fisher grade, Glasgow Coma Scale (GCS) score, and presence of hydrocephalus. Also, Glasgow Outcome Scale (GOS) at postoperative 3 months, premature rupture, ventriculostomy and LT fenestration during aneurysmal clipping, and V-P shunt after the operations were included in the analysis. Clinical status after operation was classified according to GOS. Under this rating system, a GOS score of 1 describes death; a GOS score of 2, persistent vegetative state; a GOS score of 3, severe disability (conscious but disabled); a GOS score of 4, moderate disability (disabled but independent); and a GOS score of 5, excellent recovery with return to baseline functional status (20).
The patients involved were divided into two groups. Group I (n=36) consisted of patients in whom LT fenestration was performed, Group II (n=35) consisted of patients in whom LT fenestration was not carried out. Hunt-Hess grade, Fisher grade, GCS score, presence of hydrocephalus, premature rupture, and ventriculostomy were analyzed by univariate analysis about the effect on LT fenestration. Each variable was transformed into a binary variable. Odds ratios (ORs), 95% confidence intervals (CIs) for ORs, and p-values that were calculated by use of the χ2 test were compared between Group I and Group II, and the validity of those results were analyzed by Fisher exact test. For the inspection of the intensity of correlation between LT fenestration, ventriculostomy and chronic hydrocephalus, significance; p-values, 95% confidence interval, and ORs were calculated by use of logistic regression analysis. A value of p<0.05 was considered significant; all mean values were reported as means±standard deviations.
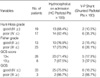
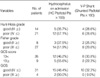
The characteristics of Group I and II are depicted in Table 1 and 2. There were no siginificant differences among the subgroups with respect to neurological scale scores (Hunt-Hess grade, GCS score, GOS) and the severity of the SAH (Fisher grade). The incidence of hydrocephalus at admission and the rate of shunting tended to increase with worsening of SAH severity (Tables 1, 2). Mean ages were 49.44±15.66 yr in Group I (n=36, 50.7%) and 54.66±13.24 yr in Group II (n=35, 49.3%). Female patients were 17 women in Group I (47.2%) and 19 women in Group II (54.3%) (Table 3).
Fisher grades were higher in Group II (3.26±0.82) than Group I (2.75±0.65) (p=0.2997; OR=0.5242) (Fig. 1A). In patients who had hydrocephalus at admission, LT openings were performed more often. Incidence of hydrocephalus was higher in Group I (75%, 27/36) than Group II (48.6%, 17/35) and it was statistically significant (p=0.0287; OR=3.176) (Fig. 1B). Correlation between LT fenestration and intraoperative ventriculostomy had a statistically significant difference of 94.4% in Group I and 28.6% in Group II (p<0.0001; OR=42.500) (Fig. 1C). LT fenestration was performed still more frequently in patients who had intraoperative ventriculostomy. Hunt-Hess grade, GCS score, and premature rupture had no significant predictive value with regard to the LT fenestration (Table 3).
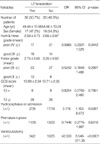
V-P shunt was undertaken from 19th day to 90th day (mean 37.3 days) after aneurysmal clipping in 18 patients (25.4%) (8 patients: 8/36 (22.2%) in Group I; 10 patients: 10/35 (28.6%) in Group II). The rate of V-P shunt had no significant difference between Group I and Group II (p=0.594; OR=0.7143) (Table 4). The rate of conversion from acute hydrocephalus on admission to shunt-dependent hydrocephalus was 29.6% (8/27) in Group I, 58.8% (10/17) in Group II, and the overall conversion rate was 40.9% (18/44).
V-P shunt was undertaken in 18 patients (25.4%) (13 patients: 13/44 (18%) in whom ventriculostomy was chosen; 5 patients: 5/27 (7%) in whom ventriculostomy was not chosen). The rate of V-P shunt had no significant difference between ventriculostomy performed and not performed groups (p=0.4026; OR=1.845) (Table 5).
Since the first report of Bagley in 1928 (21), hydrocephalus has been appraised by an important complication after the SAH and chronic hydrocephalus that required a shunt operation is reported over all in about 20% of SAH (10-13, 16). According to the development mechanism, it is thought that acute hydrocephalus is developed by a compromise of CSF circulation and/or malabsorption from arachnoid granulation due to the blood clot in the aqueduct of Sylvius, 4th ventricle, and basal cistern. In chronic hydrocephalus, fibrosis and obstruction of the subarachnoid granulation is contributing to permanent malabsorption, and promotes the communicating hydrocephalus (4, 6-9). Sometimes acute hydrocephalus can be transformed to chronic hydrocephalus, but there are some circumstances that the etiologic mechanisms are not certain. Joakimsen et al. researched the CSF hydrodynamics after SAH. They suggested the clue that there were two forms of hydrocephalus: one was the normal pressure hydrocephalus which was resulted from the normal CSF hydrodynamics and the other was the disturbed CSF hydrodynamics related hydrocephalus (13). Therefore, we can deduce that the different mechanisms may be involved in the development of the chronic hydrocephalus after aneurysmal SAH. Since it has been required a minimum of 10 days for fibrosis of leptomeninges, chronic hydrocephalus usually occurs at around 2 weeks after SAH. Therefore, close observation is recommended for more than 6 months in the high risk patients (5, 11).
The fenestration of LT has been used to treat the non-communicating hydrocephalus that accompanied elevated intracranial pressure caused by the obstructive pathologies in the midbrain and/or posterior cranial fossa structures for more than 80 yr (19). Postulated mechanisms include CSF flow through the patent LT opening to an absorptive subarachnoid space and rapid transmission of the pulse pressure through a free communicating CSF space (18). Yasargil recommended that LT fenestration should be reserved for those instances in that opening of the other basal arachnoid cisterns does not provide sufficient CSF release, especially in that case of blocked basal cistern by adherent hematomas (22). Fox and Sengupta used the LT fenestration to manage the acute hydrocephalus and/or to prevent the progression of subsequent chronic hydrocephalus (23).
Sindou reported that fenestration of the LT and Lilliequist's membrane had a favorable effect on the outcomes by facilitating CSF circulation through the basal cistern in 197 cases of the ruptured aneurysmal SAH patients (17). Tomasello et al. reported that the LT fenestration decreased the fibrosis in cisterns by promoting the hematoma toileting in the basal cistern and subarachnoid space, and improved the clinical results by reducing the incidence of chronic hydrocephalus to 4.2% much lower than mean 15-20% in the other contemporary studies (18). On the contrary, the previous reports had been studied in small series; Komotar et al. reviewed 582 cases retrospectively. They suggested that chronic hydrocephalus after anterior circulation aneurysmal SAH was the communicating type caused by an obliteration of arachnoid granulation in the cerebral convexity. On the other hand, chronic hydrocephalus after posterior circulation aneurysmal SAH was more commonly the non-communicating type, for which obstruction level was located at the outside the outflow of the fourth ventricle at the foramina of Luschka and Magendie. They also reported that LT fenestration reduced both the overall shunt rate and the rate of conversion from acute hydrocephalus on admission to shunt-dependent hydrocephalus by more than 80%, decreased the morbidity and mortality related to the shunt operation (19). The LT fenestration has been performed as a safe procedure by many surgeons (18, 19), and our experience was the same. However, transient confusion, memory loss, decreased level of consciousness, and hypothalamic injuries were reported (24) and it always had a possibility to injure vascular structures around the optic chiasm resulting in the irreversible cerebral damage.
The other studies have reported that the clinical grade on admission, the age of the patient, the amount of the SAH, aneurysm location, hyponatremia, hypertension, and the use of antifibrinolytic are known as factors associated with the development of hydrocephalus after aneurysmal SAH (1, 4, 5, 13, 15, 25, 26). As many studies had proved the statistical relationships between these factors and the development of hydrocephalus, we limited the study design to the effect of the single factor that is the LT fenestration on the development of hydrocephalus in anterior circulation aneurysmal SAH which was mainly caused by a communicating type mechanism due to subarachnoid fibrosis, and its statistical correlation was calculated by logistic regression analysis after dividing the patients into two groups according to the usage of LT fenestration (Table 4). Significant clinical criteria were analyzed by use of univariate analysis about the factors enforced the fenestration of LT during surgery (Table 3) (Fig. 1). Severity of SAH (Fisher grade) and neurological status (Hunt-Hess grade, GCS score, and GOS) did not have any significant difference between Group I and II, and the incidence of hydrocephalus on admission and the rate of shunting tend to increase with worsening SAH severity. However, the decrement trend of shunting rate is in inverse proportion to the severity of patient's status in Group II which could be caused by the effect of a confounding factor which was that the hemorrhage was so severe in poor grade patients that mortality rate before the shunt surgery increased (Table 1, 2).
We performed the LT fenestration when the patients met the criteria such as a large amount of SAH, ventricular enlargement on the CT findings, and difficulty of cerebral retraction during open craniotomy. Since the probability that accompanied the hydrocephalus at the time of surgery was higher in Group I than Group II, the incidence of hydrocephalus on admission was higher in Group I with statistical significance (p=0.0287; OR=3.176). But the Fisher grade was higher in Group II than Group I even beyond expectations (p=0.2997; OR=0.5242). Although it might be due to an inter-observer bias about the radiological criteria of Fisher grade, basic cause could be considered as a decrease of patients who were enabled to be operated on about the aneurysmal SAH due to the increased preoperative mortality in Group I (Fig. 1A).
The case where ventriculostomy was performed was accompanied by the LT fenestration during aneurysmal surgery because cerebral edema made the brain retraction difficult in most instances (p<0.0001, OR=42.500). Conclusively, when a lot of intracerebral hemorrhage made severe intracerebral parenchymal edema and/or acute hydrocephalus aggravated the cerebral edema, the fenestration of LT could help to get an adequate operative field. The rate of conversion from acute hydrocephalus on admission to shunt-dependent hydrocephalus had a difference, but the rate of V-P shunt had no significant difference between Group I and Group II (p=0.594; OR=0.7143). LT fenestration and ventriculostomy were concomitantly performed in most patients during aneurysmal surgery in Group I (p<0.0001, OR=42.500).
As mentioned above, this result, which proposed no significant correlation between the LT fenestration and progression of shunt-dependent hydrocephalus, is against those of Komotar et al. (19), Sindou (17) and Tomasello et al. (18). It is thought that the LT fenestration performed with ventriculostomy concomitantly as a routine procedure provokes the efflux of CSF from the ventricles and subarachnoid space. The reduction of effective circulatory volume of CSF resulted in a decrease of hematoma toileting from subarachnoid space during the acute phase of SAH in Group I. It is concluded that remnant hematoma caused fibrosis of the subarachnoid space, influenced the progress to the chronic hydrocephalus, and finally, the incidence of chronic hydrocephalus was not significantly different between Groups I and II. Though results of this study are opposite to the previous reports in that the LT fenestration could reduce the development of chronic hydrocephalus, this study model is not performed by prospective randomized clinical trial and is restricted to SAH patients with ACoA aneurysms because of an easy feasibility of the LT fenestration, so it is considered to have an internal limitation because of such a selection bias. In the future, it will be necessary to prove it statistically using many people as the subjects of investigation by a case-control study or prospective and retrospective cohort studies. Several reports have suggested that the LT fenestration during the aneurysmal surgery can reduce the incidence of shunt-dependent hydrocephalus (chronic hydrocephalus). However, this study provides microsurgical LT fenestration which can play a negative role for reducing the incidence of chronic hydrocephalus.
In conclusion, although the fenestration of LT can be a safe and easy procedure during ACoA aneurysm operations, the prophylactic effect for preventing the development of chronic hydrocephalus is not prominent and potential complications, including the injury of neighboring vascular structure and brain parenchyma can occur. Therefore, the neurosurgeons must give careful consideration to LT fenestration during surgery for the ruptured ACoA aneurysms.
Figures and Tables
Fig. 1
Graphs showing the distribution of patients according to variable factors. (A) Fisher grade is unexpectedly higher in Group II, and it has not statistical significance (p=0.2997; OR=0.5242). (B) Preoperative hydrocephalus and lamina terminalis (LT) fenestration is significantly associated (p=0.0287; OR=3.176). (C) In Group I, intraoperative ventriculostomy is performed in almost all cases as a routine procedure (p=<0.0001; OR=24.500).

References
1. Black PM. Hydrocephalus and vasospasm after subarachnoid hemorrhage from ruptured intracranial aneurysms. Neurosurgery. 1986. 18:12–16.

2. Mohr G, Ferguson G, Khan M, Malloy D, Watts R, Benoit B, Weir B. Intraventricular hemorrhage from ruptured aneurysm: Retrospective analysis of 91 cases. J Neurosurg. 1983. 58:482–487.
3. Vassilouthis J. The syndrome of normal pressure hydrocephalus. J Neurosurg. 1984. 61:501–509.
4. Vassilouthis J, Richardson AE. Ventricular dilatation and communicating hydrocephalus following spontaneous subarachnoid hemorrhage. J Neurosurg. 1979. 51:341–351.

5. Vale FL, Bradley EL, Fisher WS 3rd. The relationship of subarachnoid hemorrhage and the need for postoperative shunting. J Neurosurg. 1997. 86:462–466.

6. Foltz EL, Ward AA Jr. Communicating hydrocephalus from subarachnoid bleeding. J Neurosurg. 1956. 13:546–566.

7. Kosteljanetz M. CSF dynamics in patients with subarachnoid and/or intraventricular hemorrhage. J Neurosurg. 1984. 60:940–946.

8. Kibler RF, Couch RS, Crompton MR. Hydrocephalus in the adult following spontaneous subarachnoid haemorrhage. Brain. 1961. 84:45–61.
9. Torvik A, Bhatia R, Murthy VS. Transitory block of the arachnoid granulations following subarachnoid haemorrhage: A postmortem study. Acta Neurochir (Wien). 1978. 41:137–146.
10. Auer LM, Mokry M. Disturbed cerebrospinal fluid circulation after subarachnoid hemorrhage and acute aneurysm surgery. Neurosurgery. 1990. 26:804–809.

11. Gjerris F, Borgesen SE, Sorensen PS, Boesen F, Schmidt K, Harmsen A, Lester J. Resistance to cerebrospinal fluid outflow and intracranial pressure in patient with hydrocephalus after subarachnoid haemorrhage. Acta Neurochir (Wien). 1987. 88:79–86.
13. Joakimsen O, Mathiesen EB, Monstad P, Selseth B. CSF hydrodynamics after subarachnoid hemorrhage. Acta Neurol Scand. 1987. 75:319–327.

14. Choi JJ, Koh HS, Cho JH, Kim SH, Youm JY, Song SH, Kim Y. Clinical study on risk factors of hydrocephalus after aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2001. 30:1375–1380.
15. Graff-Radford NR, Torner J, Adams HP Jr, Kassell NF. Factors associated with hydrocephalus after subarachnoid hemorrhage: a report of the Cooperative Aneurysm Study. Arch Neurol. 1989. 46:744–752.

16. Gruber A, Reinprecht A, Bavinzski G, Czech T, Richling B. Chronic shunt-dependent hydrocephalus after early surgical and early endovascular treatment of ruptured intracranial aneurysms. Neurosurgery. 1999. 44:503–512.

17. Sindou M. Favourable influence of opening the lamina terminalis and Lilliequist's membrane on the outcome of ruptured intracranial aneurysms: A study of 197 consecutive cases. Acta Neurochir (Wien). 1994. 127:15–16.

18. Tomasello F, d'Avella D, de Divitiis O. Does lamina terminalis fenestration reduce the incidence of chronic hydrocephalus after subarachnoid hemorrhage? Neurosurgery. 1999. 45:827–832.

19. Komotar RJ, Olivi A, Rigamonti D, Tamargo RJ. Microsurgical fenestration of the lamina terminalis reduces the incidence of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002. 51:1403–1413.

20. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975. 1:480–484.
21. Bagley C. Blood in the CSF: Resultant functional and organic alterations in the central nervous system: A. Experimental data, B. Clinical data. Arch Surg. 1928. 17:18–81.
22. Yasargil MG. Microneurosurgery. 1984. Vol 1. Stuttgart: Georg Thieme Verlag;346–347.
23. Fox JL, Sengupta RP. Apuzzo MLJ, editor. Anterior communicating artery complex aneurysms. Brain surgery: Complication avoidance and management. 1993. Vol 1. New York: Churchill Livingstone;1009–1035.
24. Apuzzo ML, Litofsky NS. Apuzzo MLJ, editor. Surgery in and around the anterior third ventricle. Brain Surgery: Complication avoidance and management. 1993. Vol 1. New York: Churchill Livingstone;541–580.
25. Pietilä TA, Heimberger KC, Palleske H, Brock M. Influence of aneurysm location on the development of chronic hydrocephalus following SAH. Acta Neurochir (Wien). 1995. 137:70–73.

26. Säveland H, Hillman J, Brandt L, Edner G, Jackobsson KE, Algers G. Overall outcome in aneurysmal subarachnoid hemorrhage: a prospective study from neurosurgical units in Sweden during a 1-year period. J Neurosurg. 1992. 76:729–734.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download