Abstract
Hemoglobin is the predominent pigment in the gastrointestinal mucosa, and the development of electronic endoscopy has made it possible to quantitatively measure the mucosal hemoglobin volume, by using a hemoglobin index (IHb). The aims of this study were to make a software program to calculate the IHb and then to investigate whether the mucosal IHb determined from the electronic endoscopic data is a useful marker for evaluating the color of intramucosal gastric carcinoma with regard to its value for discriminating between the histologic types. We made a software program for calculating the IHb in the endoscopic images. By using this program, the mean values of the IHb for the carcinoma (IHb-C) and those of the IHb for the surrounding non-cancerous mucosa (IHb-N) were calculated in 75 intestinal-type and 34 diffuse-type intramucosal gastric carcinomas. We then analyzed the ratio of the IHb-C to the IHb-N (C/N ratio). The C/N ratio in the intestinal-type carcinoma group was higher than that in the diffuse-type carcinoma group (p<0.001). In the diffuse-type carcinoma group, the C/N ratio in the body was lower than that in the antrum (p=0.022). The accuracy rate, sensitivity, specificity, and the positive and negative predictive values for the differential diagnosis of the diffuse-type carcinoma from the intestinal-type carcinoma were 94.5%, 94.1%, 94.7%, 88.9% and 97.3%, respectively. IHb is useful for making quantitative measurement of the endoscopic color in the intramucosal gastric carcinoma, and the C/N ratio by using the IHb would be helpful for distinguishing the diffuse-type carcinoma from the intestinal-type carcinoma.
The mucosal color changes seen at endoscopy are important for making the gastrointestinal endoscopic diagnosis, not only because the color changes enable the endoscopist to detect flat or depressed intramucosal gastric carcinomas which are usually small, but also because they help to predict the degree of histologic differentiation of these cancers. The intestinal-type gastric carcinoma has the same color or reddening when compared with the surrounding non-cancerous mucosa, whereas the diffuse-type gastric carcinoma is discolored and has a paler color compared with the surrounding mucosa (1). Some pathologic studies have suggested that these color changes seen endoscopically in intramucosal gastric carcinoma seemed to be correlated with the vascularity within the carcinomatous mucosa (2). The endoscopic color of early gastric carcinoma has usually been described as reddened, the same, discolored, or pale when compared with the color of the surrounding non-cancerous mucosa (1, 2). Yet such descriptions are subjective, and it has not been possible to measure the color quantitatively.
Hemoglobin is the predominent pigment in the gastrointestinal mucosa, so measurement of the hemoglobin content is a reasonable method for quantifying the color in the gastrointestinal endoscopic images. A technique for measuring the mucosal hemoglobin content by using electronic endoscopic imaging data has been developed (3). The hemoglobin index (IHb) can be calculated by logarithmic transformation of the ratio of red color tone (R) to green color tone (G) obtained by electronic endoscopy (3).
This quantitative assessment has rarely been applied to the diagnosis of gastric cancer (4-7). If the color changes that are seen endoscopically in intramucosal gastric carcinomas are actually derived from the mucosal vascularity of the carcinomatous tissue, then it could be assumed that the measurement of the mucosal hemoglobin content would be a useful marker for a quantitative assessment of the endoscopic color of gastric carcinoma.
If the IHb in the regions of interest of the endoscopic image could be automatically calculated by a software program, it might be very helpful to measure color quantitatively. However, to the best of our knowledge, there has been no software program to calculate the IHb in the regions of interest.
Accordingly, the aims of this study were to make a software program to calculate the IHb and then to investigate whether the mucosal IHb determined from the electronic endoscopic data is a useful marker for evaluating the color of intramucosal gastric carcinoma with regard to its value for discriminating between the histologic types.
The electronic endoscopic images of 109 intramucosal gastric carcinomas from 109 consecutive patients were studied retrospectively from January 2003 to May 2005. The patients had no anemia, congestive heart failure or portal hypertensive gastropathy. All the carcinomas were of the superficial type (Type 0-II) according to the macroscopic classification of the Japanese Research Society of Gastric Cancer (8). Seventy-nine carcinomas were resected surgically and 30 were resected endoscopically. All the carcinomas were confirmed to be restricted to the gastric mucosa and the ulceration with exudates was excluded by histopatholgic investigation. The 109 gastric carcinomas were divided into two groups according to Lauren's classification (9): 75 intestinal-type and 34 diffuse-type. The median diameters of the intestinal-type and diffuse-type were 12 mm (range 2 to 60 mm) and 25 mm (range 4 to 70 mm), respectively.
The electronic endoscopic system consisted of an EVIS-240 and EVIS LUCERA videoscope system (Olympus Optical Co., Ltd., Tokyo, Japan) with a magnetic optical disk drive. An electronic upper endoscope such as GIF-Q240, GIF-Q260 or GIF-H260 (Olympus Optical Co., Ltd., Tokyo, Japan) was connected to the system and the white balance was readjusted for calibration of red, green, and blue signals prior to each examination. Any mucus on the gastric mucosa or on the lens was removed by careful washing with water before the endoscopic image was obtained. A directly facing view of the lesion was obtained after extension by insufflated air at a distance of 2 to 6 cm from the lesion (3, 4). The electronic endoscopic images were recorded in the database system without any compression or image processing, following analogue-to-digital conversion by 24-bit color image capture board during the endoscopic examination.
The IHb was calculated for each pixel of the electronic endoscopic images by logarithmic transformation of the Vr/Vg ratio using the following equation: IHb=32[log2 (Vr/Vg)] (3). In the above equation, Vr indicates the signal brightness for red (wavelength near 650 nm, showing minimal absorption by Hb) and Vg indicates the signal brightness for green (wavelength near 560 nm, showing maximal absorption by Hb).
For the medical image processing, the image areas of interest need to be selected from the input images for the accurate and fast processing. Four method types are currently used for the area selection: the user-defined type, the doughnut-type, the rectangle-type and the line-type (Fig. 1). In the user-defined selection method, users can draw the boundary of an area of interest by using the moving mouse, so that the area closest to a user-wanted is able to be selected. The user-defined method displays the selected area by detecting and linearly connecting the mouse position per 1/100 sec. Thus, a rugged and polygonal area is selected if users move the mouse quickly, while a fine and detailed area may be selected if users move the mouse slowly. In the doughnut-type method, users first draw an area of user-defined shape and then draw another area of a wanted shape in the inside or outside of the area, consequently generating two areas, the inner area and the doughnut-type area. The doughnut-type method is able to separately analyze the area of interest and the surrounding area and then support the comparison between the two areas. In the rectangle-type method, if users mark the start point and the end point of an area of interest by using the mouse, the rectangle area fitting the diagonal line between the two points is selected. In the line-type method, if users mark the start and the end points of a line across the interested area, an area of belt-type including the line is displayed.
For the analysis of a selected area, the analysis system is able to take a shape for each color channel and display the results of the statistical analysis such as histograms, mean values and median values for the color channels, etc. Also, the system supports the area segmentation into smaller areas along with color channels.
Generally, a selected area is displayed using the RGB (Red, Green and Blue) color model in the computer, but conversion into the IHb color model is required for medical image analysis, because the distribution of the red color can be analyzed better on the IHb color model. Also, for the analysis of the color distribution in a selected area, the color mean value Ravg and the color median value Rmid are calculated, and the various information of color distribution for each color channel is displayed.
The distribution information for color channel is represented with histograms, mean values and median values for the 3 channels of RGB color model and the IHb channel (Fig. 2).
The area segmentation function supported by the analysis system provides a picture-style representation of the color distribution in a selected area by segmenting the area into smaller areas along with the color channels. This function can also provide the information on the position of some color channels and the color value of a diagnostic area, etc.
For the area segmentation function, some options such as the color model and the segmentation method are provided. In the color model option, the selection of the IHb model is more efficient than the RGB model for medical image analysis, and in the segmentation method option, one of the following methods can be selected: segmentation using the mean value, segmentation using the median value, segmentation using the user-defined value and segmentation using the user-defined number of segments (Fig. 3).
For the analysis of a predefined line, the analysis system displays enlargement of the line and represents the color distribution of the line as histograms, mean values and standard deviations for the color channels.
The enlargement of the line makes possible visual recognition of the change of the color values in the line at some level, and the histogram for each color channel depicts the change of the color channel value along with the line in the whole. In addition, the mean value Lavg and the standard deviation Lsd for each color channel are represented together with the histogram, and this improves the ease of analysis.
Without the information of the histologic type, one gastroenterologist calculated the mean values of the IHb for the carcinoma (IHb-C) and the mean values of the IHb for the surrounding non-cancerous mucosa (IHb-N) in each of the regions of the interest. We mainly used the doughnut-type method for the area selection (Fig. 4, 5) but this was impossible in 37 endoscopic images. At that time, we used the user-defined method. Then, we analyzed the ratio of the IHb-C to the IHb-N (C/N ratio).
All results were expressed as mean±SD. Comparison of the C/N ratio was carried out using the Mann-Whitney U test. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, U.S.A.) program version 11.0. Differences with a p value of less than 0.05 were considered to be statistically significant.
The mean C/N ratio in the intestinal-type carcinoma group was higher than that in the diffuse-type carcinoma group: 1.28±0.19 vs. 0.81±0.18, respectively (p<0.001, Fig. 6). According to the location of the carcinoma, there was no difference in the C/N ratio between the antrum and the body in the intestinal-type carcinoma group (1.27±0.16 vs. 1.32±0.25, respectively, p=0.421). But in the diffuse-type carcinoma group, the C/N ratio in the body was lower than that in the antrum (0.72±0.18 vs. 0.86±0.16, respectively, p=0.022, Fig. 7).
The C/N ratio was in excess of 1.0 in 94.7% (71/75) of the intestinal-type carcinoma group. In contrast, the C/N ratio was below 1.0 in 94.1% (32/34) of the diffuse-type carcinoma group. Especially the C/N ratio was below 1.0 in all 13 diffuse-type carcinomas in the body (Table 1).
When the cut-off point of the C/N ratio was set at 1.00 (10), the accuracy rate, sensitivity, specificity, and the positive and negative predictive values of a C/N ratio below 1.00 for the differential diagnosis of the diffuse-type carcinoma from the intestinal-type carcinoma were 94.5%, 94.1%, 94.7%, 88.9% and 97.3%, respectively.
In this study, the measurement of the IHb calculated from the electronic endoscopic imaging data was used to quantify the endoscopic color of the gastric carcinoma. We showed that the intestinal-type carcinoma group had a higher C/N ratio than the diffuse-type carcinoma group. We also showed that the C/N ratio was in excess of 1.0 in 94.7% (71/75) of the intestinal-type carcinoma group and 5.9% (2/44) of the diffuse-type carcinoma group. These results were similar to a previous report (6) and this fact suggests that the C/N ratio obtained by using the IHb is of clinical relevance in distinguishing between the intestinal-type carcinoma and the diffuse-type carcinoma.
From a clinical point of view, the pretreatment distinction between the intestinal-type and the diffuse-type intramucosal gastric carcinoma is of particular importance because it directly influences the choice of endoscopic versus surgical resection (11-13). The established indications for endoscopic resection of early gastric carcinoma are as follows: 1) differentiated carcinoma (i.e. intestinal-type carcinoma), 2) size 2 cm or less, 3) restricted to the mucosa, and 4) absence of ulceration, where there is no possibility of lymph node metastasis (11-14). If the gastric carcinoma fulfills all of these criteria, endoscopic resection is the therapy of choice for early gastric carcinoma because this modality maintains a good quality of life and has a favorable survival rate that is comparable with surgical resection (12, 13). Early gastric carcinoma has been assessed using both radiologic and endoscopic modalities (15-18), that is, the size is assessed radiologically and the depth of invasion is assessed by a combination of conventional endoscopy and endosonography. Although it is possible to suggest the histologic type of carcinoma by a simple description of the endoscopic color of lesion, it is very subjective. Yao et al. firstly reported an objective method for evaluating the endoscopic color of the early gastric cancer and for discriminating the histologic type of carcinoma by using the C/N ratio of the IHb (6).
In the endoscopic diagnosis of small flat or depressed intramucosal gastric carcinoma, the morphologic characteristics such as irregular and polygonal shape, clear demarcation, and irregular margins are important (19, 20). The diagnosis of depressed-type intramucosal gastric carcinoma is relatively easy on gross inspection through careful endoscopic assessment (20). From the standpoint of a scientific approach to endoscopic diagnosis, we can stress that an objective value (the C/N ratio of the IHb) for standardizing endoscopic color is highly superior to any subjective or descriptive expressions such as reddened, slightly reddened, the same or discolored as compared with the surrounding mucosa.
In the previous pathologic studies, the vascularity of gastric carcinoma within the mucosa was shown to differ according to the degree of the histologic differentiation (2, 21, 22). It was found that the mucosal vascularity of differentiated carcinoma compared with the surrounding non-cancerous mucosa was higher or about the same in most lesions, but lower in a few. However, the mucosal vascularity of most undifferentiated carcinomas was hypovascular compared with the surrounding non-cancerous mucosa. These differences in the vascularity have been explained as being due to the differences in the microvascular architecture of the carcinomatous tissue. Intramucosal differentiated carcinoma is often accompanied by a proliferation of vessels within the neoplastic interstitial tissue, while the individual carcinoma cells of intramucosal undifferentiated carcinoma infiltrates discretely and destroys the normal mucosal vascular architecture without any proliferation of the interstitial tissue. Consequently, differences in mucosal vascularity contribute to the endoscopic color change of intramucosal gastric cancer, that is, the reddened color of the differentiated gastric carcinoma derives from increased mucosal vascularity, whereas the paler color of the undifferentiated gastric carcinoma is due to the reduced vascularity.
In vivo, several factors such as ulceration influence the vascularity of the gastric mucosa (4). For the purpose of determining the amount of mucosal hemoglobin that is characteristic of carcinomatous mucosa, we investigated the intramucosal gastric carcinomas that were not accompanied by ulceration with exudates.
We showed that a C/N ratio (below 1.00) of the IHb had a high accuracy rate (94.5%) for the diffuse-type intramucosal gastric carcinoma and this result was similar to a previous report (6). This fact could be useful in the selection of patients who should not undergo endoscopic resection. A prospective study about the clinical efficacy of the IHb such as application to the indication of endoscopic resection in early gastric carcinoma would be needed.
Our results were similar to the report of Yao et al. (6). However, there is a difference in the analytic method between their study and our study. They analyzed the linear region of the carcinoma and the surrounding non-cancerous mucosa but there was the possibility of error. First, the linear region of a carcinoma is very small portion of a carcinoma and it does not represent the whole region of a carcinoma. Second, the C/N ratio of the IHb could be changed according to the selection of the linear region. For example, if there is a flat carcinoma with focally reddish and focally whitish lesion, the C/N ratio of the IHb may be increased when analyzing the reddish lesion but it may be decreased when analyzing the whitish lesion. In this study, we analyzed the area of the carcinoma and the surrounding mucosa by mainly using the doughnut-type method. Because we included more regions, we could avoid the above mentioned errors.
Yet we still had some limitations in analyzing the area of the carcinoma and the surrounding mucosa. First, sometimes we could not include the whole area of the carcinoma in one endoscopic image due to large size or the problem of location. In this situation, we selected the region of interest by using the user-defined method. Second, the border of carcinoma that we marked could be somewhat different from the true border of the carcinoma in some cases. But the different degree between the marked border and the true border of carcinoma would be small if any, so this would not influence our results.
The endoscopic color of the body is somewhat different from that of the antrum. So, we analyzed the C/N ratio according to the location of the carcinoma. There was no difference of the C/N ratio between the antrum and the body in the intestinal-type carcinoma group. But in the diffuse-type carcinoma group, the C/N ratio in the body was lower than that in the antrum. Generally the body has redder endoscopic color, i.e. higher IHb-N, than the antrum. This fact might somewhat explain the difference of the C/N ratio according to the location in the diffuse-type carcinoma group. Further studies would be required to answer why the difference according to the location was present only in the diffuse-type carcinoma group. Also, a prospective study about the clinical efficacy of the IHb such as application to the indication of endoscopic resection in early gastric carcinoma will be needed.
In conclusion, the IHb is useful for quantitative measurement of the endoscopic color in intramucosal gastric carcinoma and the C/N ratio by using the IHb would be helpful in distinguishing the diffuse-type carcinoma from the intestinal-type carcinoma.
Figures and Tables
Fig. 2
Display of an example of the distribution information for each color channel in a selected area.
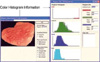
Fig. 4
(A) Electronic endoscopic image of an intestinal-type gastric carcinoma in the body of the stomach. The first marking was done on the margin of the carcinoma and then the second marking was done on the surrounding non-cancerous mucosa. (B) The mean IHb was calculated from the data of the picture elements on the described area. The C/N ratio was 1.26 (IHb-C: 37.7, IHb-N: 30.0).
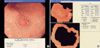
Fig. 5
(A) Electronic endoscopic image of a diffuse-type gastric carcinoma in the body of the stomach. The first marking was done on the margin of the carcinoma and then the second marking was done on the surrounding non-cancerous mucosa. (B) The mean IHb was calculated from the data of the picture elements on the described area. The C/N ratio was 0.84 (IHb-C: 28.3, IHb-N: 33.6).
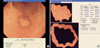
Fig. 6
The C/N ratio in the diffuse-type carcinoma group and in the intestinal-type carcinoma group.

References
1. Misumi A, Misumi K, Murakami A, Harada K, Honmyo U, Akagi M. Endoscopic diagnosis of minute, small, and flat early gastric cancers. Endoscopy. 1989. 21:159–164.
2. Honmyo U, Misumi A, Murakami A, Mizumoto S, Yoshinaka I, Maeda M, Yamamoto S, Shimada S. Mechanisms producing color change in flat early gastric cancers. Endoscopy. 1997. 29:366–371.

3. Tsuji S, Sato N, Kawano S, Kamada T. Functional imaging for the analysis of the mucosal blood hemoglobin distribution using electronic endoscopy. Gastrointest Endosc. 1988. 34:332–336.

4. Tsuji S, Kawano S, Hayashi N, Tsujii M, Ogihara T, Kamada T, Sato N. Analysis of mucosal blood hemoglobin distribution in gastric ulcers by computerized color display on electronic endoscopy. Endoscopy. 1991. 23:321–324.

5. Sato N, Tsuji S, Kawano S, Hayashi N, Tsujii M, Ishigami Y, Ogihara T, Nagano K, Kamada T. Computer-assisted two-dimensional analysis of gastric mucosal hemoglobin distribution using electronic endoscopy. Endoscopy. 1992. 24:Suppl 2. 522–526.

6. Yao K, Yao T, Matsui T, Iwashita A, Oishi T. Hemoglobin content in intramucosal gastric carcinoma as a marker of histologic differentiation: a clinical application of quantitative electronic endoscopy. Gastrointest Endosc. 2000. 52:241–245.

7. Yao K, Kato M, Fujisaki J. Techniques using the hemoglobin index of the gastric mucosa. Endoscopy. 2005. 37:479–486.

8. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 2nd English Edition. Gastric Cancer. 1998. 1:10–24.
9. Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histoclinical classification. Acta Pathol Microbiol Scand. 1965. 64:31–49.
10. Browner WS, Newman TB, Cummings SR. Hulley SB, Cummings SR, editors. Designing a new study: III. Diagnostic test. Designing clinical research. 1988. 1st ed. Baltimore: Williams & Wilkins;87–97.
11. Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg. 1992. 79:241–244.

12. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003. 57:567–569.

13. Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993. 25:445–450.

14. Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, Endo Y, Hosokawa K, Saito D, Shim CS, Gossner L. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005. 37:178–182.

15. Sano T, Okuyama Y, Kobori O, Shimizu T, Morioka Y. Early gastric cancer. Endoscopic diagnosis of depth of invasion. Dig Dis Sci. 1990. 35:1340–1344.
16. Yanai H, Matsumoto Y, Harada T, Nishiaki M, Tokiyama H, Shigemitsu T, Tada M, Okita K. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointest Endosc. 1997. 46:212–216.

17. Akahoshi K, Chijiiwa Y, Hamada S, Sasaki I, Maruoka A, Kabemura T, Nawata H. Endoscopic ultrasonography: a promising method for assessing the prospects of endoscopic mucosal resection in early gastric cancer. Endoscopy. 1997. 29:614–619.

18. Akahoshi K, Chijiiwa Y, Hamada S, Sasaki I, Nawata H, Kabemura T, Yasuda D, Okabe H. Pretreatment staging of endoscopically early gastric cancer with a 15 MHz ultrasound catheter probe. Gastrointest Endosc. 1998. 48:470–476.

19. Oohara T, Aono G, Ukawa S, Takezoe K, Johjima Y, Kurosaka H, Asakura R, Tohma H. Clinical diagnosis of minute gastric cancer less than 5 mm in diameter. Cancer. 1984. 53:162–165.

20. Oiwa T, Mori M, Sugimachi K, Enjoji M. Diagnostics of small gastric carcinoma. J Surg Oncol. 1986. 33:170–175.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download