Abstract
To facilitate the establishment of mixed chimerism with limited dose of bone marrow (BM) cells, and to achieve tolerance in skin graft model, combined blocking of costimulatory pathway and IL-2 pathway was used in minimally myeloablative model using busulfan. BM cells (2.5×107) of BALB/c were injected into C57BL/6 mice at day 0 with full thickness skin graft after single dose injection of busulfan (25 mg/kg) on day -1. Recipients were grouped and injected the anti-CD154, CTLA4-Ig, anti-IL-2R at days 0, 2, 4, and 6 according to protocol. Mixed macrochimerism were induced in groups treated with anti-CD154+anti-CTLA4-Ig, anti-CD154+anti-IL-2R, and anti-CD154+anti-CTLA4 Ig+anti-IL-2R. Three groups having chimerism enjoyed prolonged graft survival more than 6 months. Superantigen deletion study revealed deletion of alloreactive T cells in combined blockade treated groups. In graft versus host disease model using CFSE staining, CD4+ T cell and CD8+ T cell proliferation were reduced in groups treated with CTLA4-Ig or anti-IL-2R or both in combination with anti-CD154. However, anti-IL-2R was not so strong as CTLA4-Ig in terms of inhibition of T cell proliferation. In conclusion, IL-2 pathway blocking combined with anti-CD154 can establish macrochimerism with limited dose of BM transplantation and induce specific tolerance to allograft.
Effective immunosuppression without any adverse reaction is one of the most important objective of organ transplantation. Calcineurin inhibitor based immunosuppression regimens have been widely used and improved graft survival with reducing the episode of acute rejection. However, it may have serious complications such as life-threatening infections and increased incidence of cancer. New strategies are still needed to obtain the elusive goal of donor-specific immunosuppression and tolerance by the host to toward foreign graft. Therefore, achieving immune tolerance is regarded as the goal in the field of transplantation.
Allograft rejection requires robust T cell activation and proliferation. For the full activation of T cells, costimulation is necessary. Blockade of the CD28-B7 and/or CD40-CD154 pathways significantly prolonged allograft survival of both rodents and primate models by reducing acute allograft rejection (1-10). Although many successful results of prolonging allograft survival in murine models were reported, some strains of mice such as C57BL/6 showed relatively resistant to these types of treatment and allograft rejection with near normal kinetics (11-13). IL-2 plays an important role in clonal expansion and continuing viability of activated T lymphocytes. Depleting IL-2 or blocking its receptor (IL-2R) can reduce the acute rejection and enhance the allograft survival (14-16). However, single use of IL-2 pathway blocking agent is not sufficient to delay acute rejection and improve graft survival (17). Thus, IL-2 pathway blocking methods were used in combination with other immunosuppressive modalities such as calcineurin inhibitors in clinical transplantation or with costimulation blocking agent and reported prolonged allograft survival even in costimulation blockade resistant strain (11).
A proven method to produce robust transplantation tolerance is the induction of donor hematopoietic chimerism. Some previous studies had reported successful and prolonged allograft survival using this method (18, 19). However, it needs large dose of irradiation or cytoablative agents such as cyclophosphamide to make niche for the engraftment of donor bone marrow (BM) cells in recipient bone marrow. Recently, costimulation blocking agents were used to reduce radiation dose and drugs and some studies reported prolonged graft survival (20-23). Using costimulatory blocking agents combined with administration of megadoses of non-T cell depleted bone marrow achieved chimerism without pretransplant conditioning (21, 23). However, large dose of bone marrow under protection of costimulatory blocking agent is not suitable for the clinical application in the setting of deceased donor transplantation since the total number of BM cells are limited. Use of busulfan in combination with costimulatory blockade was introduced to reduce toxicity and induce titratable degrees of macrochimerism to induce tolerance without cytoreductive preconditioning (20, 24). Until now, most of the studies have used anti-CD154 mAb and cytotoxic T-lymphocyte antigen 4 immunoglobulin (CTLA-Ig) as costimulation blockades, and this combination treatment with BM transplantation always achieved stable chimerism in different studies (20-24).
We postulated that simultaneous blockade of costimulatory pathway with anti-CD154 and IL-2 pathway followed by donor bone marrow transplantation could promote titrable, high-level of macrochimerism with single dose of busulfan and limited numbers of bone marrow cells and could achieve at least comparable results to anti-CD154 and CTLA4-Ig treatment. If it is successful, it could be easily applicable in clinical settings with less toxicity of conventional immunosuppression and induce specific tolerance to donor organs resulting in prolonged graft survival.
Adult male BALB/c (H-2d) and C57BL/6 (H-2b) mice, each weighing 20 g were obtained from Orient Co. (Sungnam city, Korea) and housed in specific pathogen free conditions under the guidance of institutional guidelines for animal care and welfare.
Bone marrow cells were obtained from tibiae, femurs, and humeri of BALB/c mice using sterile saline flush. After lysis of red blood cells (RBC) with Trizma base ammonium chloride (TBAC) solution, single cell suspensions were made and resuspended at 2.5×107 cells/0.4 mL of sterile saline and injected via penile vein of recipient mice (C57BL/6) on day 0. For recipient preparation, busulfan (Busulfex®, Jeil-Kirin Pharm. Inc., Seoul, Korea) (25 mg/kg) was administered on day -1. Anti-IL-2R mAb (PC61 clone [ATCC:TIB 222]: 250 µg/day), hamster anti-mouse CD154 mAb (MR1; 500 µg/day) and CTLA4-Ig (500 µg/day) were administered on days 0, 2, 4, and 6. All the antibodies were purchased from Bioexpress Co. (Lebanon, NH, U.S.A.). Recipient mice were grouped by treatment regimens, such as BM+busulfan, group I: BM+busulfan+anti-CD154, group II; BM+busulfan+anti-CD154+anti-IL-2R, group III; BM+busulfan+anti-CD154+CTLA Ig, group IV; and BM+busulfan+anti-CD 154+CTLA4 Ig+anti-IL-2R, as group V.
Full thickness skin grafts (1 cm2) of dorsum of BALB/c ear were transplanted on dorsal thorax of C57BL/6 mice on day 0 after injection of bone marrow cells, and secured with Band-Aid® (Johnson & Johnson, Arlington, TX, U.S.A.) for 7 days. After removal of Band-Aid, graft survival was observed everyday. Rejection was defined as complete loss of graft tissue.
Whole peripheral blood of recipients was obtained from tail vein and stained with flurorochrome-conjugated antibody (Abs) (anti-CD3e, anti-H-2Kd, anti-H-2Kb, anti-CD25, anti-CD4, anti-Vβ8.1/8.2, anti-Vβ5.1/2, anti-Vβ11; Becton Dickinson, San Diego, CA, U.S.A.) followed by RBC lysis and washing with whole blood lysis kit (R&D System, Minneapolis, MN, U.S.A.). Stained cells were analyzed with Cell Quest software on a FACScaliber flow cytometer (Becton Dickinson, Mountain View, CA, U.S.A.).
T cells from each recipient group were obtained with a Pan T cell isolation kit (Miltenyi Biotec, Auburn, CA, U.S.A.) followed by RBC lysis using TBAC solution (pH 7.6) from recipient spleens and resuspended on 5 µM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugine, OR, U.S.A.) solution to attach CFSE probes. Single cell suspension labeled with CFSE (2×107 cells) was injected into a vein of BALB/c mice which had been supralethally irradiated with 1,800 rad of total dose. After 66 hr, spleens from BALB/c mice were procured and single cell suspension was obtained with same manner as described above. Those single cell suspensions were stained with anti-CD4 and anti-CD8 Ab (Caltag, Burlingame, CA, U.S.A.) and analyzed by flow cytometry.
We examined the ability of single dose of busulfan and BM cells to promote hemopoietic chimerism in allograft model. After two weeks from bone marrow transplantation, we had checked the formation of mixed chimerism and ratio from peripheral blood. The mixed chimerism was described as percent ratio of H-2Kd positive T cells to total CD3e+ T cells. Mixed chimerisms were rarely shown in group I (0.78±0.20%) and group II (6.50±0.71%). However, in group III, IV and V, we found relatively high ratio of mixed chimerism formation at two weeks (34.23±8.31%, 24.48±3.03%, 24.54±2.68%). The ratio of bone marrow chimerism increased until 4 months after the bone marrow transplantation, then, became plateau for whole periods of experiment with range of 65% to 75% ratio (Fig. 1). From this result we confirmed that anti-CD154 alone could not achieve chimerism with limited dose of BM cells, and addition of anti-IL-2R worked synergistically. Anti-IL-2R alone could not induce chimerism in preliminary experiment (data not shown).
Donor specific allograft survival was observed using skin graft to see if chimeric animal accept graft without rejection. In group I, which received bone marrow only, could not prolong graft survival (median survival time [MST], 11 days). Anti-CD154 alone prolonged graft survival modestly (MST, 21 days), however, all the grafts failed eventually with limited dose of bone marrow. On the contrary, graft survival of group III, IV and V, to which anti-IL-2R or CTLA4-Ig or both were added, showed graft survival of more than 6 months (Fig. 2). Anti-IL-2R alone could not prolong graft survival and rejected rapidly as in control group in preliminary experiment (data not shown).
In normal condition, BALB/c mice delete Vβ11 and Vβ5.1/2 positive CD4+ T cells in the thymus due to their high affinity for endogenous retroviral superantigens (mouse mammary tumor virus [MMTV]) presented by I-E MHC class II molecules, whereas C57BL/6 mice do not express I-E and utilize 4-5% of Vβ11 positive CD4+ T cells and 2-3% of Vβ5.1/5.2 positive CD4+ T cells. Both BALB/c and C57BL/ 6 mice have 15-20% of CD4+ T cells which express Vβ8.1/8.2 (37, 38). Six months after the skin graft, we checked alloreactive T cell deletion of each group using flow cytometric analysis (Fig. 3). The group II, which had been administered anti-CD154 only, has both CD4+ Vβ11+ T cells (3.80±1.50%) and CD4+ Vβ5+ T cells (1.14±0.73%). In group III, IV, and V which had additional anti-IL-2R or CTLA4 Ig or both, CD4+ T cells which express Vβ11+ and Vβ5+ were deleted almost completely compared to normal level (Vβ11; 1.05±0.35% vs. 0.79±0.21% vs. 0.94±0.34%, Vβ5; 0.39±0.16% vs. 0.25±0.13% vs. 0.54±0.23%). However, CD4+ Vβ8+ T cells in all groups showed persistently. This means that chimeric BALB/c cells deleted donor reactive recipient T cells in the thymus.
To evaluate if the donor specific T cell proliferation is inhibited, T cells were harvested from recipient groups and control mice 2 months after transplantation. CFSE stained T cells were administrated to supralethally irradiated BALB/c mice (1,800 rad). After 66 hr of in vivo proliferation, T cells were harvested from spleens of BALB/c mice and flow cytometric analysis was performed followed by anti-CD4 and anti-CD8 stain. As a result, proliferation of T cells was minimal in groups IV and V. Anti-IL-2 R further suppressed the proliferation of T cells in group III compared to group II, but it could not fully prevent T cell division from allostimulation. In group V, CTLA4-Ig already inhibited T cell proliferation and anti-IL-2R had no additional effect on that. CD4+ and CD8+ T cells were fully proliferated in the other groups and normal control (Fig. 4).
The development of clinically applicable strategies to induce a state of mixed hematopoietic chimerism for the treatment of genetic hematologic diseases and to induce a state of specific immunological tolerance to organ allograft has received increasing attention over the past several years. After initial reports by Sachs and colleagues (25, 26), many studies reported the therapeutic potential of hematopoietic chimerism to induce tolerance in adult animal models (27-29). In spite of many successful reports, there were some problems concerning the potential for over immunosuppression, loss of memory (non specific peripheral T cell depeletion), and/or enhanced risk of malignancy with whole body irradiation which may limit the clinical applications of these protocols (20). To reduce the risk of preconditioning, supraphysiological doses of non-T cell-depleted donor bone marrow transplantation method under costimulation blockade was developed and induced a robust state of donor specific tolerance (21, 23). Although these methods could avoid the toxicity of preconditioning, they also have problems in using very high numbers of BM cells which would be impractical in clinical practice.
Undepleted T cells have increased the risk of lethal graft versus host disease (GVHD), and relatively low level of chimerism was achieved (23). Although GVHD could be inhibited by the use of costimulation blockade, megadoses of T cells were necessary to achieve titrable macrochimerism. Because of these reasons, these protocols seem to be clinically unacceptable for deceased donor organ transplantation in which the available amounts of bone marrow cells are limited (20). Theoretically, using T cell-depleted BM cells is better than using whole BM cells. However, we could achieve good level of chimerism as other reports which used T cell-depleted BM cells (20). Durham et al. (21) also could achieve similar level of chimerism without any evidence of GVHD in repeated injection of BM cells experiment. In the recent report of Adams et al. (24), they also used non-T cell-depleted BM cells and achieved similar level of chimerism. Actually, it was found in preliminary experiment that the level of chimerism was similar between non T cell-depleted BM transplantation group and T cell-depleted BM transplantation group (data not shown).
There were several trials to make niche in the bone marrow to facilitate the engraftment of transplanted BM cells, such as irradiation or using cytotoxic drugs (22, 28). However, irradiation achieved more than 90% of chimerism even in very small amount of irradiation, which was just like substitution of recipient bone marrow with donor BM cells. Recently, busulfan was introduced as a regimen which makes stable chimerism (20). Busulfan has superiority to irradiation since it does not show any residual effect. Furthermore, it has less toxicity than irradiation and preserves white blood cell counts better than irradiation (20). Previous studies used single dose of busulfan (day 5) and two doses of BM cells (days 0 and 6) (20, 24). However, in clinical situation, donor bone marrow is not enough for two dose and also difficult to keep for a week. Therefore, we tried to use limited numbers of BM cells as single dose injection with minimal preconditioning using single dose of busulfan (25 mg/kg) one day before administration of BM cells.
Anti-CD154 only could not induce stable chimerism in this study, which was different from high dose BM cell infusion report in which BM cells were infused up to 90 days (21). Therefore, large numbers of BM cells are necessary to induce chimerism in situation with limited blocking of costimulation signals. Although anti-IL-2R alone could not achieve titrable BM chimerism (data not shown), BM chimerisms were successfully induced in groups III, IV, and V. Therefore, anti-IL-2R and CTLA4-Ig synergistically worked with anti-CD154 to induce stable chimerism. Ratio of BM chimerism was increased by 4 months and maintained with range of 65 to 75%. This mixed chimerism was stable until the end of experiment (more than 300 days). This result is identical with previous megadose BM cell infusion study or two dose BM cell infusion study (20, 21, 24). As other reports, combined use of anti-CD154 and CT- LA4-Ig seems to be perfect match to induce stable chimerism and graft tolerance (20, 24). However, CTLA4-Ig is patented product and preclinical and clinical studies are still on going. On the other hand, anti-IL-2R is used in clinics everyday, and using anti-IL-2R is at least comparable to using CTLA4-Ig in combination with anti-CD154. Therefore, it would be easier to use anti-IL-2R than CTLA4-Ig when other costimulation blockade would be available.
In previous report by Jones et al., IL-2 pathway blocking combined with costimulation blockade was able to enhance graft survival in a costimulation resistant rejection model (11). Although Dai et al. reported that, in transplant setting, IL-2 plays important role in the elimination of alloreactive T cells by enhancing activation induced cell death (30), combined use of anti-IL-2R and costimulation blockade effectively blocked the generation of alloreactive T cells (11). However, without BM cell infusion, blocking of IL-2 pathway combined with anti-CD154 and CTLA4-Ig could not achieve tolerance and failed the graft eventually.
Clonal deletion of donor specific alloreactive T cells is corner stone of robust immune tolerance and it is the main mechanism of inducing central tolerance. To compare the donor specific deletion, we compared the utilization of Vβ11, Vβ5.1/2, and Vβ8.1/2 by CD4+ T cells in B6 recipients among groups. As previously expressed, BALB/c BM cells and combined blocking of costimulation signals deleted Vβ11- and Vβ5.1/2- bearing T cells. Our data show that the clonal deletion of alloreactive T cells is the main mechanism of inducing tolerance in BM mixed chimerism model, which is consistent with other reports (20, 21, 24).
Another major problem in inducing BM mixed chimerism is GVHD. Ferrara et al. reported that in vivo administration of anti-IL-2R can ameliorate the acute GVHD in bone marrow transplantation (31). In our data, CFSE staining analysis showed that the proliferation of CD4+ T cell and CD8+ T cell were absent in groups treated with CTLA-4 Ig and anti-IL-2R in GVHD model. Combined use of costimulation blockade inhibited the proliferation of alloreactive T cells which was also a part of mechanism of tolerance induction. IL-2 pathway blocking only is less effective in preventing allograft rejection. Similar results in different models were reported in previous studies from Jones and others (11). Although combined use of anti-CD154 and anti-IL-2R prolonged graft survival and showed stable mixed chimerism, the proliferation study showed a certain portion of proliferated alloreactive T cells. This may suggest that this group should be observed very long time to see if they regain alloreactivity, or there is an operational tolerance or ignorance even with the presence of alloreactive T cells. There is also a possibility that active regulation of T cells with regulatory T cells might exist. From the reports that blocking CD154-CD40, CD28-B7 and IL-2R pathways result in down regulation of T cell proliferation and induce regulatory T cell proliferation, we could recognize that the proliferation of regulatory T cell is controlled by costimulatory molecules (32-34). This should be explored in next experiment. Although anti-IL-2R are not strong enough to block the proliferation of alloreactive T cells than CTLA4-Ig, it showed comparable results in terms of graft survival with using CTLA4-Ig.
Although further studies using large animals and primates should follow, we can utilize mixed chimerism model with minimal myeloablation strategies and limited dose of BM cells to prolong the graft survival and induce tolerance. Combined use of costimulation blockade together with anti-IL-2R could be a nontoxic regimen in inducing tolerance.
Figures and Tables
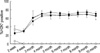 | Fig. 1Mixed chimerism was measured from peripheral blood of C57BL/6 recipients having BALB/c BM cells. Percentages of donor-derived T cells to the total number of T cells were expressed. Groups are allogeneinc bone marrow transplantation with busulfan only (△; n=6), treated with anti-CD154 (□; n=4), anti-CD154 and anti-IL-2R (○; n=5), anti-CD154 and CTLA4-Ig (■; n=8), anti-CD154, anti-IL-2R and CTLA-4 Ig (●; n=8). |
 | Fig. 2Survival of skin allografts. Combined use of costimulation blockade achieved indefinite graft survival. Groups are allogeneinc bone marrow transplantation with busulfan only (△; n=6), treated with anti-CD154 (□; n=4), anti-CD154 and anti-IL-2R (○; n=5), anti-CD154 and CTLA4-Ig (■; n=8), anti-CD154, anti-IL-2R and CTLA-4 Ig (●; n=8). |
 | Fig. 3The utilization of Vβ5, Vβ8 and Vβ11 by CD4+ T cells in peripheral blood of control (C57BL/6, BALB/c) and treated mice. Allogeneic bone marrow transplant with busulfan. C57BL/6 recipients were treated with anti-CD154 (n=6), anti-CD154 and anti-IL-2R Ab (n=6), anti-CD154 and CTLA4-Ig (n=8), anti-CD154, anti-IL-2R and CTLA4-Ig (n=8). |
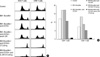 | Fig. 4(A) Representative samples from a flow cytometric assay for T cell proliferation in vivo. Supralethally irradiated BALB/c mice received CFSE-labeled T cells from naïve and treated mice. Cells were harvested at 66 hr after infusion. (B) Percentage of precursor A cells which divided 4 or more times. |
References
1. Rossini AA, Greiner DL, Mordes JP. Induction of immunologic tolerance for transplantation. Physiol Rev. 1999. 79:99–141.

2. Uchida N, Shirasugi N, Akiyama Y, Matsumoto K, Shimazu M, Kitajima M, Hamano K, Aramaki O, Ikeda Y, Niimi M. Induction of indefinite survival of fully allogeneic cardiac grafts and generation of regulatory cells by intratracheal delivery of alloantigens under blockade of the CD40 pathway. Transplantation. 2003. 75:878–884.

3. Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting Edge: Anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000. 165:4783–4786.

4. Niimi M, Pearson TC, Larsen CP, Alexander DZ, Hollenbaugh D, Aruffo A, Linsley PS, Thomas E, Campbell K, Fanslow WC, Geha RS, Morris PJ, Wood KJ. The role of the CD40 pathway in alloantigen-induced hyporesponsiveness in vivo. J Immunol. 1998. 161:5331–5337.
5. Kenyon NS, Fernandez LA, Lehmann R, Masetti M, Ranuncoli A, Chatzipetrou M, Iaria G, Han D, Wagner JL, Ruiz P, Berho M, Inverardi L, Alejandro R, Mintz DH, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes. 1999. 48:1473–1481.

6. Elster EA, Xu H, Tadaki DK, Burkly LC, Berning JD, Baumgartner RE, Cruzata F, Patterson NB, Harlan DM, Kirk AD. Primate skin allotransplantation with anti-CD154 monotherapy. Transplant Proc. 2001. 33:675–676.

7. Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, Fechner JH Jr, Germond RL, Kampen RL, Patterson NB, Swanson SJ, Tadaki DK, TenHoor CN, White L, Knechtle SJ, Harlan DM. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999. 5:686–693.

8. Sayegh MH, Zheng XG, Magee C, Hancock WW, Turka LA. Donor antigen is necessary for the prevention of chronic rejection in CTLA4Ig-treated murine cardiac allograft recipients. Transplantation. 1997. 64:1646–1650.
9. Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994. 57:1701–1706.
10. Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995. 181:1869–1874.

11. Jones TR, Ha J, Williams MA, Adams AB, Durham MM, Rees PA, Cowan SR, Pearson TC, Larsen CP. The role of the IL-2 pathway in costimulation blockade-resistant rejection of allografts. J Immunol. 2002. 168:1123–1130.

12. Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1+ CD8+ T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999. 104:1715–1722.

13. Guo Z, Meng L, Kim O, Wang J, Hart J, He G, Alegre ML, Thistlethwaite JR Jr, Pearson TC, Larsen CP, Newell KA. CD8 T cell-mediated rejection of intestinal allografts is resistant to inhibition of the CD40/CD154 cotimulatory pathway. Transplantation. 2001. 71:1351–1354.
14. Kirkman RL, Barrett LV, Gaulton GN, Kelley VE, Ythier A, Strom TB. Administration of an anti-interleukin 2 receptor monoclonal antibody prolongs cardiac allograft survival in mice. J Exp Med. 1985. 162:358–362.

15. Kupiec-Weglinski JW, Diamantstein T, Tilney NL, Strom TB. Therapy with monoclonal antibody to interleukin 2 receptor spares suppressor T cells and prevents or reverses acute allograft rejection in rats. Proc Natl Acad Sci USA. 1986. 83:2624–2627.

16. Reed MH, Shapiro ME, Strom TB, Milford EL, Carpenter CB, Weinberg DS, Reimann KA, Letvin NL, Waldmann TA, Kirkman RL. Prolongation of primate renal allograft survival by anti-Tac, an anti-human IL-2 receptor monoclonal antibody. Transplantation. 1989. 47:55–59.

17. MacLean JA, Su Z, Guo Y, Sy MS, Colvin RB, Wong JT. Anti-CD3: anti-IL-2 receptor bispecific monoclonal antibody. Targeting of activated T cells in vitro. J Immunol. 1993. 150:1619–1628.
18. Nakamura T, Good RA, Yasumizu R, Inoue S, Oo MM, Hamashima Y, Ikehara S. Successful liver allografts in mice by combination with allogeneic bone marrow transplantation. Proc Natl Acad Sci USA. 1986. 83:4529–4532.

19. Ikehara S, Ohtsuki H, Good RA, Asamoto H, Nakamura T, Sekita K, Muso E, Tochino Y, Ida T, Kuzuya H, Imura H, Hamashima Y. Prevention of type I diabetes in nonobese diabetic mice by allogenic bone marrow transplantation. Proc Natl Acad Sci USA. 1985. 82:7743–7747.

20. Adams AB, Durham MM, Kean L, Shirasugi N, Ha J, Williams MA, Rees PA, Cheung MC, Mittelstaedt S, Bingaman AW, Archer DR, Pearson TC, Waller EK, Larsen CP. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol. 2001. 167:1103–1111.

21. Durham MM, Bingaman AW, Adams AB, Ha J, Waitze SY, Pearson TC, Larsen CP. Cutting edge: administration of anti-CD40 ligand and donor bone marrow leads to hemopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J Immunol. 2000. 165:1–4.

22. Brodsky I, Bulova S, Crilley P. The role of busulfan/cyclophosphamide regimens in allogeneic and autologous bone marrow transplantation. Cancer Invest. 1989. 7:509–513.

23. Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, Shaffer J, Sayegh MH, Sykes M. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000. 6:464–469.

24. Adams AB, Shirasugi N, Jones TR, Williams MA, Durham MM, Ha J, Dong Y, Guo Z, Newell KA, Pearson TC, Larsen CP. Conventional immunosuppression is compatible with costimulation blockade-based, mixed chimerism tolerance induction. Am J Transplant. 2003. 3:895–901.

25. Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984. 307:168–170.

26. Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989. 169:493–502.

27. Markees TG, Philips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4+ T cells, interferon γ and CTLA4. J Clin Invest. 1998. 101:2446–2455.
28. Tomita Y, Sachs DH, Sykes M. Myleosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994. 83:939–948.
29. Shehee WR, Oliver P, Smithies O. Lethal thalassemia after insertional disruption of the mouse major adult β-globin gene. Proc Natl Acad Sci USA. 1993. 90:3177–3181.
30. Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Impaired alloantigen-mediated T cell apoptosis and failure to induce long-term allograft survival in IL-2-deficient mice. J Immunol. 1998. 161:1659–1663.
31. Ferrara JL, Marion A, McIntyre JF, Murphy GF, Burakoff SJ. Amelioration of acute graft vs host disease due to minor histocompatibility antigens by in vivo administration of anti-interleukin 2 receptor antibody. J Immunol. 1986. 137:1874–1877.
32. Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000. 12:431–440.

33. Kumanogoh A, Wang X, Lee I, Watanabe C, Kamanaka M, Shi W, Yoshida K, Sato T, Habu S, Itoh M, Sakaguchi N, Sakaguchi S, Kikutani H. Increased T cell autoreactivity in the absence of CD40-CD40 ligand interactions: a role of CD40 in regulatory T cell development. J Immunol. 2001. 166:353–360.

34. Suzuki K, Nakajima H, Saito Y, Saito T, Leonard WJ, Iwamoto I. Janus kinase 3 (Jak3) is essential for common cytokine receptor gamma chain (gamma(c))-dependent signaling: comparative analysis of gamma(c), Jak3, and gamma(c) and Jak3 double-deficient mice. Int Immunol. 2000. 12:123–132.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download