Abstract
We evaluated the risk of coronary-artery disease in patients with chronic renal failure (CRF) by measuring the coronary-artery calcium scores with electron beam CT (EBCT). A total of 81 CRF patients were divided into three groups; pre-dialysis (group I, n=35), hemodialysis (group II, n=31) and peritoneal dialysis (group III, n=15). The several serum biochemical markers and calcium score levels by EBCT were determined. The Ca×P products were significantly higher in groups II (p<0.05) and III (p<0.01) than in group I. The serum calcium levels were significantly higher in group III than in both group I (p<0.01) and II (p<0.05). The serum calcium level in 15 patients with a calcium score > 400 was significantly higher than the 66 patients with a score ≤400 (p<0.01). The calcium score was significantly higher in the 15 patients with cardiovascular complications than in the 66 patients without cardiovascular complications (628.9±904.8 vs. 150.4±350.9, p<0.01). EBCT seemed to be a good diagnostic tool for evaluating the risk of coronary-artery disease "non-invasively" in CRF patients who are at increased risk of cardiovascular morbidity and mortality.
There is a high likelihood of mortality in patients with end stage renal disease (ESRD), who are susceptible to ischemic heart disease, due to cardiovascular disease more frequently than ordinary people (1), and approximately 50% of these patients die from cardiovascular disease (2, 3). Goodman et al. (4) emphasized the clinical importance of the early detection of coronary artery atherosclerosis by reporting that calcification of the coronary artery in those young patients (approximately 20-30 yr of age) on dialysis due to ESRD is more likely to occur than in ordinary people. Moreover, it has been reported that the severe calcification seen in subjects with ESRD actually begins long before renal insufficiency is severe enough to require dialysis (5).
Recently, electron beam computed tomography (EBCT) was introduced as a method for evaluating the extent of coronary artery calcification in a non-invasive way (6). The coronary artery calcium score is the result of a quantitative evaluation on the calcified area of the coronary artery using EBCT. The critical condition for obstructive coronary disease is more likely to increase if the degree of calcification inside the coronary artery is high (i.e., the calcium score is high) (7). Rumberger et al. (8) reported that patients with a calcium score >400 by the EBCT calcium score guidelines are very likely (≥90%) to have at least one "significant" coronary artery stenosis.
We undertook this study to determine the prevalence and extent of coronary-artery calcification in predialysis, hemodialysis, and peritoneal dialysis patients and also to evaluate the usefulness of EBCT among chronic renal failure (CRF) patients at risk for cardiovascular diseases.
The study involved 81 patients who suffered from CRF, who were followed up for pre-dialysis receiving conservative treatment, hemodialysis (HD), or peritoneal dialysis (PD) in Kyungpook National University Hospital (Daegu, Korea) from July 2000 to March 2004. The patients were divided into pre-dialysis (Group I, n=35), HD (Group II, n=31), and PD groups (Group III, n=15). All subjects gave informed consent before participation, and the study was approved by the Ethics Committee of Kyungpook University Hospital.
Screening for coronary-artery calcification was performed using EBCT. The serum hemoglobin, albumin, blood urea nitrogen (BUN), creatinine (Cr), total cholesterol, calcium (Ca), phosphorus (P), and parathyroid hormone (PTH) levels were measured simultaneously. The existence of cardiovascular complications, primary disease of the CRF, the duration of dialysis, age, and sex were determined. The age and duration of dialysis were standardized by the termination point of the EBCT and blood test. The primary diseases of the CRF with the exception of chronic glomerulonephritis, diabetes mellitus, and hypertension were classified as "Others". Cases with a history of cerebrovascular accidents, peripheral vascular disease, and coronary artery disease were classified as "cardiovascular complications".
All imaging procedures were performed using EBCT (C-150 E.B.T., Imatron, CA, U.S.A.). Contiguous transverse imaging sections were obtained with using a 3 mm collimation. The images were reconstructed with using a 260 mm or 300 mm field of view, a matrix of 512 by 512, and a sharp reconstruction filter. All scans were scored with using specialized software called "Insight Calcium Plaque Analysis Program" by a skilled physician. According to Agatston et al. (9), the overall calcium score for each patient was counted by the sum total of the multiplication of the area of coronary artery calcium comprised of at least 4 consecutive pixels in more than 130 HU (Hounsfield Unit) CT density, by the coefficient from 1 to 4, as determined by the peak CT density of each lesion.
The values are given as mean±SD in case of a normal distribution or otherwise a median and range. The distribution of the categorical variables among the groups was assessed by chi-square analysis. The comparisons among the groups were made using Mann-Whitney and Kruskal-Wallis tests. A p value <0.05 was considered as statistically significant.
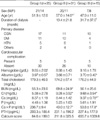
There were 48 of men and 33 of women whose ages ranged from 16 to 76 yr with an average age 53. The proportion of males to females in group I, II and III was 21:14, 20:11 and 7:8, respectively. The mean age was higher in group II than in groups I and III (57.0±14.0 vs. 51.9±12.0 and 47.9±11.0 yr, p<0.05). The duration of dialysis was longer in group III on PD than in group II on HD (31.7±37.0 vs. 13.4±21.8 months, p<0.05). The primary disease of the CRF included 38 cases (47%) of chronic glomerulonephritis followed by 25 cases (31%) of diabetes mellitus and 12 cases (15%) of hypertension. The other diseases included 3 cases of polycystic kidney disease, one renal tuberculosis, and 2 unknown causes. The cardiovascular complications amounted to 15 (19%) out of a total 81 patients, which included 9 coronary artery disease cases, 5 cerebrovascular accidents and one peripheral vascular disease. There were 5 patients with history of cardiovascular complications in each group (Table 1).
Although the Ca×P product as well as the BUN and Cr of group II were significantly higher than in group I, there was no distinctive difference in the calcium score including the PTH, Ca and P. In a comparison between group I and III, the Ca and Ca×P product as well as the Cr in group III were significantly higher than in group I. In a comparison between group II and III, the serum Ca in group III was significantly higher than group II with no significance in the other parameters. The calcium score in groups I, II and III obtained from EBCT was 84.6±199.0 (range, 0 to 903; median, 4), 211.8±325.5 (range, 0 to 1054; median, 30) and 655.7±1009.8 (range 0 to 3356; median, 16), respectively, in which group III showed higher results. However, there was no statistical significance (Table 1).
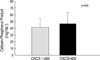
When a total of 81 patients were divided into a dialysis (group II and III, n=46) and pre-dialysis group (group I, n=35), the serum albumin level of the pre-dialysis group was significantly higher than in the dialysis group (3.97±0.57 vs. 3.67±0.62 g/dL, p<0.05). The BUN, Cr, P (5.35±1.71 vs. 4.48±1.36 mg/dL, p<0.05) and Ca×P product (46.6±15.3 vs. 36.7±9.4 mg2/dL2, p<0.01) of the dialysis group were significantly higher than in the pre-dialysis group. However, other biochemical parameters were not significantly different in both groups. The calcium score of the dialysis group was 356.6±657.4. This was higher than that in the pre-dialysis group, which was 84.6±199.0. However, there was no statistical significance (Table 2).
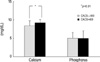
The P and PTH levels in the patients (n=15) with a calcium score >400 and in those (n=66) with a calcium score ≤400 were not significantly different. However, the Ca×P product tended to be higher and the serum Ca level was significantly higher (9.22±0.81 vs. 8.43±1.31 mg/dL, p<0.01) in the patients with a calcium score >400 than in those with a calcium score ≤400 (Fig. 1, 2, 3).
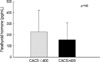
While the Ca, P, Ca×P product and PTH levels were similar between the groups with and without cardiovascular complications (n=15, n=66, respectively), the calcium score of the group with cardiovascular complications had a significantly higher level (628.9±904.8 vs. 150.4±350.9, p<0.01) (Fig. 4).
There have been many explanations for the cause of the high death rate due to cardiovascular disease in patients with CRF including hypertension, insulin resistance, dyslipidemia, and hyperhomocysteinemia (10-15). However, such existing risk factors are unable to explicate the coronary artery atherosclerosis with the accompanying complications. Block et al. (16) recently asserted that hyperphosphatemia is the risk factor, which was incurred by a metabolic disorder of Ca and P. The decrease in renal clearance and nephron, a functional unit of the kidney, leads to hyperparathyroidism by incurring hyperphosphatemia, which causes metastatic calcification on internal organs such as the myocardium, the liver, lung as well as the soft tissues, joints, vessels by increasing the Ca×P product and renal osteodystrophy from the damage to the osseous tissues. In addition, patients may die of cardiovascular disease such as a myocardial infarction if the cardiovascular calcification inclusive of coronary artery calcification is in progress. Excessive calcium overload may also be a factor for cardiovascular calcification. Goodman et al. (4) reported that the total quantity of calcium contained in the phosphate binders taken into the CRF patients of the group revealing calcification was twice that of the non-calcification group. PTH also contributes to the risk factors for cardiovascular disease, not only functioning in increasing the Ca×P product, but also causing microcalcification by an in-flowing calcium abnormally into cytoplasm (17, 18).
Magnetic resonance imaging (MRI), conventional CT, helical CT, and intravascular ultrasonography have been used to evaluate coronary artery calcification. However, calcification measurements on the coronary artery through EBCT are becoming popular. The major feature of EBCT is that it is optimized for visualizing especially the coronary artery in a high temporal resolution between 50-100 msec and in a high spatial resolution less than 0.5 mm2 (19). In addition, it enables a quantitative evaluation of coronary-artery calcification, manifested in the so called "calcium score", by sensitively and rapidly detecting the calcified lesion of all the coronary artery areas without using contrast media. Agatston et al. (9) proved that EBCT was excellent quantitative evaluation diagnostic methodology for investigating coronary artery calcification. The American Heart Association has acknowledged the validity of EBCT for detecting coronary artery calcification, where the detection of coronary artery calcification through EBCT provides the corroborative confirmation of the existence of atherosclerotic plaque in the coronary arteries. Furthermore, an increase in calcification increases the risk of obstructive coronary disease (7).
Although the major reason for the death of CRF patients is cardiovascular disease, it has been thought of as being the case only for the older ESRD patients on dialysis. However, Braun et al. (20) reported that the frequency of coronary artery calcification in the patients with ESRD on HD is larger than in age and gender matched non-dialysis patients with documented or suspected coronary artery disease and Merjanian et al. (5) reported that nondialyzed individuals with diabetic renal disease have a significantly greater prevalence and severity of vascular and valvular calcification compared to matched nondiabetic controls with normal renal function. Coronary and aortic wall calcification is significantly greater among individuals with diabetic renal disease, when compared to normoalbuminuric diabetics. This demonstrates that the severe calcification seen in subjects with ESRD actually begins long before renal insufficiency is severe enough to require dialysis. Goodman et al. (4) examined coronary artery calcification using EBCT on 39 patients with renal failure under dialysis and 60 normal controls, and performed a comparative analysis on the clinical characteristics and chemical tests on the serum Ca and P level. They reported that 14 out of 16 patients with CRF had calcification in their coronary arteries. In contrast, only 3 of the 60 in the control group had calcification. The group found to have calcification was generally older with a longer dialysis term than the non-calcification group, and the serum P, Ca×P product were also higher. In addition, 10 patients who had calcification in progress underwent EBCT again after an average 20±3 months follow-up. The results showed that the calcium score had increased approximately two times compared to the previous result. Moreover, Eifinger et al. (21) measured the extent of coronary artery calcification, using EBCT on a total of 16 asymptomatic patients ranging from 14 to 39 yr of age under renal replacement therapy. Of these, 6 patients were found to have calcification, which emphasized that the early detection of coronary artery calcification is one of the ways to prevent the cardiovascular complications and ultimately to decrease the death rate.
It might be suspected that continuous ambulatory peritoneal dialysis (CAPD) would present fewer problems with respect to renal osteodystrophy than HD does, because CAPD provides more consistent steady-state biochemical control and permits a less restrictive diet. However, the influence that the mode of dialysis has on the calcium and phosphorus metabolism of patients with renal disease is controversial. So, to determine the influence of the mode of dialysis on the cardiovascular system and calcium metabolism, our study performed the comparison by dividing the CRF patients into three groups, "Pre-dialysis" (group I, n=35), "HD" (group II, n=31), and "PD" (group III, n=15) without setting a control group and limiting the age, the duration of dialysis, and the existence of symptoms. One study (22) reported that no significant differences were seen in serum levels of calcium, phosphorus, and PTH between patients on CAPD and patients on HD. However, in our study, the serum Ca level of the PD group was significantly higher than the pre-dialysis and HD groups and the Ca×P product of the PD group tended to be higher than the HD group. In addition, when the pre-dialysis group was compared to the HD and PD groups together as a dialysis group, serum P level and the Ca×P product of the dialysis group was significantly higher than in the predialysis group and the calcium score of the dialysis group (356.6±657.4) was higher than that in the pre-dialysis group (84.6±199.0) but there was no statistical significance. While there was a tendency for the calcium score of the PD group to be higher than the pre-dialysis and HD group, there was no statistical significance, which was attributed to the small sample size of the PD group. Despite the fact that the patients in the PD group had a tendency to be younger than the predialysis and HD group, the calcium score of the PD group was higher than in the pre-dialysis and HD. It is believed that Ca, P and the Ca×P product should be controlled more strictly in the PD group than in the other groups.
Rumberger et al. (8) suggested the so-called "EBCT calcium score guidelines" in order to apply the calcium score obtained from the EBCT to clinical practice. These guidelines recommend an examination on ischemic heart disease through a pharmacological or exercise stress test together with a revision of the risk factors for those whose calcium score >400 because the risk of coronary artery disease increases up to 90% in these patients. In our study, when 66 patients with a calcium score ≤400 were compared to the 15 patients with a score >400, the group with a calcium score >400 had a significantly higher serum calcium level and tended to be higher the Ca×P product than the group with a calcium score ≤400. In addition, in a comparison between the 15 patients with cardiovascular complications and the 66 patients without such complications, the calcium score was significantly higher in those with cardiovascular complications. Our results agree with the previous evidences that arterial calcification has been linked to an elevated Ca×P product (20), and increased myocardial calcium content and vascular calcifications in dialysis patients were shown to have a strong positive correlation with an elevated Ca×P product and inverse correlation with left ventricular function (23). It has been known that elevated Ca×P product may contribute to the high mortality and morbidity of patients with end-stage renal disease. Moreover, it is certainly conceivable that vascular and cardiac calcification in particular leads to complications and increased mortality. In conclusion, this study suggests that EBCT seemed to be a good diagnostic tool for evaluating the risk of coronary-artery disease "non-invasively" in CRF patients who are at increased risk of cardiovascular morbidity and mortality.
Figures and Tables
 | Fig. 4Distribution of the coronary-artery calcium score (CACS) level according to presence or absence of cardiovascular complications (CVC). |
References
1. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998. 32:S112–S119.

2. Bloembergen WE. Cardiac disease in chronic uremia: epidemiology. Adv Ren Replace Ther. 1997. 4:185–193.

3. US Renal Data System: USRDS 1999 Annual Data Report. Bethesda, MD: The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases;and www.med.umich.edu/usrds.
4. Goodman WG, Goldin J, Kutzon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000. 342:1478–1483.

5. Merjanian R, Budoff M, Adler S, Berman N, Mehrotra R. Coronary artery, aortic wall, and valvular calcification in nondialyzed individuals with type 2 diabetes and renal disease. Kidney Int. 2003. 64:263–271.

6. Yun YS, Rhee YM, Sim DK, Sin SK, Park BK, Rhu DR, Han SH, Park SW, Song YD, Lim SK, Kim KR, Lee HC, Rim SJ, Cho SY, Huh KB, Choi KO, Lee JH. Coronary artery calcification quantified by electron beam tomography as a screening for coronary artery disease in asymptomatic non-insulin-dependent-diabetes mellitus. Korean J Med. 1999. 56:317–328.
7. Wexler L, Brundage B, Crouse J, Detrano R, Fuster V, Maddahi J, Rumberger J, Stanford W, White R, Taubert K. Coronary artery calcification: pathophysiology, epidemiology, imaging methods and clinical implication: a statement for health professionals from the American Heart Association. Circulation. 1996. 94:1175–1192.
8. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999. 74:243–252.

9. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990. 15:827–832.

10. Avram MM, Goldwasser P, Burrell DE, Antignani A, Fein PA, Mittman N. The uremic dyslipidemia: a cross-sectional and longitudinal study. Am J Kidney Dis. 1992. 20:324–335.

11. Bostom AG, Shemin D, Lapane KL, Sutherland P, Nadean MR, Wilson PW, Yobum D, Bausserman L, Tofler G, Jacques PF, Selhub J, Rosenberg JH. Hyperhomocysteinemia, hyperfibrinogenemia, and lipoprotein (a) excess in maintenance dialysis patients: a matched case-control study. Atherosclerosis. 1996. 125:91–101.

13. Mailloux LU, Haley WE. Hypertension in the ESRD patient: Pathophysiology, therapy, outcomes, and future directions. Am J Kidney Dis. 1998. 32:705–719.

14. Owen WF, Madore F, Brenner BM. An observational study of cardiovascular characteristics of long-term end-stage renal disease survivors. Am J Kidney Dis. 1996. 28:931–936.

15. Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P. "U" curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998. 54:561–569.

16. Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium×phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998. 31:607–617.
17. Faubert PF, Shapiro WB, Porush JG, Chou SY, Gross JM, Bondi E, Gomez-Leon G. Pulmonary calcification in hemodialyzed patients detected by technetium-99m diphosphonate scanning. Kidney Int. 1980. 18:95–102.

18. Massry SG. The toxic effects of parathyroid hormone in uremia. Semin Nephrol. 1983. 3:306–328.
19. Moshage W, Achenbach S, Daniel WG. Novel approaches to the non-invasive diagnosis of coronary-artery disease. Nephrol Dial Transplant. 2001. 16:21–28.

20. Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996. 27:394–401.
21. Eifinger F, Wahn F, Querfeld U, Pollok M, Gevargez A, Kriener P, Gronemeyer D. Coronary artery calcifications in children and young adults treated with renal replacement therapy. Nephrol Dial Transplant. 2000. 15:1892–1894.

22. Suzuki T, Kanno Y, Nakamoto H, Okada H, Sugahara S, Suzuki H. Peritoneal dialysis versus hemodialysis: a five-year comparison of survival and effects on the cardiovascular system, erythropoiesis, and calcium metabolism. Adv Perit Dial. 2003. 19:148–154.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download