Abstract
Seven hundred forty seven cases of gastrointestinal stromal tumors (GISTs) in Koreans who were diagnosed between 2001 and 2002 were analyzed to evaluate their occurrence and their clinical, pathologic and immunohistochemical findings. The most frequent location of tumor was in the stomach (63%), followed by the small intestine (30%), the colorectum (5%), and the esophagus (2%). c-kit expression was found in 93.6% of the cases, while CD34, SMA and S-100 protein was positive in 80.1%, 28.2%, and 20.2%, respectively. c-kit positivity was high in the stomach (94.2%) and small intestine (94.6%), while it was relatively low in the colorectum (85.0%), and esophagus (81.2%). The positivity for CD34 was correlated with the higher risk of GISTs (p=0.04). Follow up of the patients showed that 58 primary GISTs patients died and 20 of these patients were recurrent or metastatic at the time of diagnosis. The pathologic diagnosis to predict the risk of aggressive behavior of GISTs was correlated with the numbers of tumor, clinical stage, epithelioid histologic type, cellularity, cellular atypia, necrosis, and mucosal invasion (p=0.00). GISTs with a poor prognosis were closely related to the clinical stage at presentation, the locations of the tumor, and the ages of the patients.
Gastrointestinal stromal tumor (GIST) is a mesenchymal tumor of the digestive tract showing lineage differentiation similar to the interstitial cell of Cajal (ICC) and accounts for about 2% of all GI tract neoplasms (1). Their morphology varies from undifferentiated to differentiated tumors having myoid or neural differentiation. So, various kind of diagnosing methods including immunohistochemical stains and electron microscopy were established. And also recent rapid progress has been accomplished for the molecular genetics of GISTs, and studies on the molecular-based classification of GISTs are underway (2).
c-kit (CD117) is a transmembrane tyrosine kinase receptor and its expression appears to play a key role in committing the primitive mesenchymal cells towards ICC differentiation. Some GISTs contain activating mutations in the kit proto-oncogene and this is associated with the expression of the c-kit protein (3). The identification of the mutations that are mostly in exon 11 and to a lesser extent in exons 9 and 13 of the kit proto-oncogene coding for c-kit in many GISTs has resulted in a better understanding of their oncogenic mechanisms. The true neural and smooth muscle neoplasms of the GI tract entirely lack these kit mutations (4). So, c-kit expression has been proposed as being the most sensitive and specific phenotypic marker of GISTs. Although 90% of GISTs were found to be immunohistochemically positive for c-kit (5-12), and the mutations of kit gene have been found in 80% to 85% of these c-kit positive GISTs, a minority of GISTs lack any kit mutations, but nonetheless, c-kit is strongly activated (2, 4, 8). Such GISTs might contain kit mutations that are not readily detected by the conventional screening methods, or alternately, the c-kit might be activated by nonmutational mechanisms. Moreover, in 35% of the GISTs lacking the kit mutations, intragenic mutations in the related receptor for tyrosine kinase, platelet-derived growth factor receptor alpha (PDGFRA), have recently been reported (13, 14). Therefore, the current definition of GISTs as c-kit positive mesenchymal tumors of uncertain malignant potential fails to include a number of GIST cases that have similar histology. Although confirmatory c-kit staining is required for drug therapy and approximately 95% of GISTs stain positively for c-kit (15), the most important tool needed to diagnose GIST is still hematoxylin and eosin-stained section (16), and this will encompass the PDGFRA mutation-positive and c-kit-negative GISTs, both the PDGFRA and kit mutation-negative GISTs, and the rare kit mutation-positive and c-kit-negative GISTs (2).
Immunohistochemical stainings for the other markers are more variable; these include CD34 (47-100%), muscle specific actin (0-50%, usually focal staining), S-100 protein (5-30%), and desmin (2-5%) (15-17). Despite the recent remarkable progress in understanding and treating GISTs, pathologists still have difficulty for predicting behavior of GISTs. Of those GISTs that undergo resection, about half of the cases have possibility of recurrence or metastasis (18). Although a large tumor size and high mitotic activity have been strongly associated with malignancy (17), it can not completely exclude their malignant potential in tumors with small size and no mitosis (19).
However, in Korea, the incidence and the clinicopathologic features of GISTs have not been characterized because most of the previous studies have been mainly focused on one institution's experience (20-24), and the multi-institutional studies were focused on the stomach (25, 26). In an attempt to survey the approximate incidence, clinicopathologic characteristics and immunophenotypic features of GISTs in Korean, we conducted a clinicopathologic and immunohistochemical analysis of 747 mesenchymal tumors that had the histologic features of GIST.
Tissue samples were obtained from Korean patients who had mesenchymal tumors of the gastrointestinal tract. The patients who met the following criteria were selected for study; 1) they were operated or biopsied from 1 January 2001 to 31 December 2002; 2) the clinical information for this clinicopathologic study was available; 3) the patients could be followed up after the surgery or biopsy. Paraffin blocks of the GISTs were taken from 50 major hospitals in Korea including 40 university hospitals. The data sheets including the operation titles, the organ site of involvement, sizes of the tumors, number of the tumors, clinical or pathologic staging, histopathologic tumor types, mitotic counts, presence of cellular pleomorphism, benign cellular patterns, necrosis and mucosal invasion, were distributed to all participating centers of this study. All available H&E stained slides were reviewed and the important pathologic findings were carefully checked and described in each case. The diagnostic criteria for the malignant behavior of GISTs were assessed by the criteria of the NIH GIST workshop (Table 1) (17).
Immunohistochemical staining was performed with using primary antibodies including rabbit polyclonal anti-human c-kit (Dako, 1:400), CD34 (clone Qbend10, Dako, 1:50), alpha-smooth muscle actin (SMA) (clone 1A4, Dako, 1:50), and S-100 protein (polyclonal, Dako, 1:1,000) and with using a DAKO EnVision™+ System according to the manufacturer's instructions. Diaminobenzidine (DAB) was used as a chromogen. Boiling the slides in the citrate buffer for 10 min was performed with microwaves for antigen retrieval of the c-kit and CD34. The GISTs were selected on the basis of their histopathologic features and the immunohistochemical staining results for c-kit, CD34, SMA and S-100. The diagnostic criteria are summarized in Table 2. Briefly, all the mesenchymal tumors with c-kit positivity were diagnosed as GISTs, and the c-kit -negative and CD34-positive cases or all the immunomarker-negative cases having the histopathologic features of GISTs were diagnosed as "consistent with" GISTs. According to the immunophenotypic features, the tumors were classified into four types by using Rudolph et al. classification with slight modification (16): a) gastrointestinal fibrous tumors (GIFT), which were vimentin and/or CD34 positive without smooth muscle or neural differentiation; b) GISTs with smooth muscle differentiation that were SMA positive (GILT); c) GISTs with neural differentiation that were S-100 protein-positive (GINT); and d) GISTs with dual smooth muscle and neural differentiation (GIDT). The collected data were analyzed using SPSS software and by employing χ2 and Kruskal-Wallis tests and Pearson's correlation coefficient.
Seven hundred forty seven GISTs were diagnosed among the 849 gastrointestinal mesenchymal tumors that occurred from 2001 to 2002 (334 in 2001 and 413 in 2002). The male to female ratio was 374 to 373. The patients with GISTs ranged in age from 10 to 87 yr (mean 56.3 yr) and the peak age was between 50 and 70 (Fig. 1). According to the operation method, wide resection and excision were performed in 541 cases (71%), and wedge resection or biopsy were performed in 247 cases (20%); and 59 cases (8%) were found incidentally. All incidentally found GISTs were single tumors with sizes ranging from 0.2 cm to 9.5 cm, and most of them were less than 2.0 cm with the mean size being 1.2 cm. They were found during operations for gastric carcinoma in 54 cases, esophageal carcinoma in 2 cases, colon carcinoma in 2 cases and ovarian carcinoma in 1 case.
The sizes of the mass varied from 0.2 cm to 53 cm with the mean size being 6.1 cm (Fig. 2). The most frequent location of the GISTs was in the stomach, followed by the small intestine and the colorectum (Fig. 3). In 11 cases, multiple tumor masses were found after metastatic or recurrent tumor nodules had been excluded. Among these multiple GISTs, eight cases occurred in the small intestine and one case was associated with neurofibromatosis.
The clinical stages of the disease are described in Fig. 4. We found three cases of GIST showing the regional lymph nodes metastasis at the time of operation. These three cases were all male patients. The tumor displayed a high risk of malignant behavior, the mean tumor size was 12.5 cm and the tumors occurred in the small intestine, rectum and stomach, respectively. Although these three cases showed metastasis and recurrent tumor during the follow up, the patients are still alive at more than 36 months after chemotherapy.
The patients' survival status was followed up for 12-36 months by using the data obtained from the Korea National Statistical Office. Seventy one patients had died by December 31, 2003, and 13 of them had incidentally found GISTs during their operations for disease other than the GISTs. Of the remaining 58 primary GISTs, the small intestine and the stomach were the most common locations with 28 and 25 cases, respectively, while the colorectum and the esophagus comprised the remaining 5 cases. Among 58 patients who died of GISTs, 20 of them showed recurrent or metastatic tumor nodules at the time of diagnosis. Their pathologic diagnosis included 50 high risk tumors, 4 intermediate risk tumors and 4 low risk tumors. Forty nine (84.5%) of the 58 cases showed c-kit protein expression. In this study, prognosis of the GISTs were closely related to the clinical stages at presentation, pathologic diagnosis of GISTs, and the locations (p<0.05).
The breakdown of the low risk GISTs patients having a poor prognosis showed 2 small intestinal, 1 esophageal and 1 rectal tumor location with the patients' ages being over 65 yr; there were multiple tumors in 2 cases and diffuse cytoplasmic staining for c-kit protein in 3 cases.
Of the total 849 cases of mesenchymal tumors of the gastrointestinal tract, 747 cases were diagnosed as GISTs including 48 GIST-like tumors that met the criteria described in the materials and methods section. When all the GISTs were classified according to the pathologic factors that define the risk of aggressive behavior in GISTs, very low risk, low risk, intermediate risk and high risk were found in 112 (13.6%), 216 (29.1%), 159 (21.4%), and 255 (34.4%) cases, respectively. The increasing aggressive risk of GISTs was correlated with increased number of tumors, higher clinical stage, epithelioid histology, high cellularity, severe cellular atypia, the presence of necrosis, and mucosal invasion (p=0.00).
For histology, spindle cell type was found in 578 cases (77.4%) (Fig. 5A), epithelioid cell type was found in 66 cases (8.8%) (Fig. 5B), and mixed type was found in 103 cases (13.8%) (Fig. 5C). Benign cellular patterns such as hyaline changes (Fig. 5D), cystic changes and myxoid changes (Fig. 5E) were observed in 267 cases, which included 46 cases of very low risk, 97 cases of low risk, 48 cases of intermediate risk and 76 cases of high risk. The benign cellular pattern was inversely correlated with the risk level of the GISTs (p=0.007). Necrosis (Fig. 5F) was found in 215 cases, of which 2 cases were very low risk, 24 cases were low risk, 38 cases were intermediate risk and 151 cases were high risk. The low risk group showing necrosis exhibited sizes around 5 cm and mitotic figures close to 5/50HPF. Mucosal invasion (Fig. 5G) was found in 149 cases; there were 2 cases of very low risk, 26 cases of low risk, 34 cases of intermediate risk, and 87 cases of high risk. Dystrophic calcification was found in 2 cases of GISTs with low and very low risk of malignant potential, respectively. Skeinoid fibers (Fig. 5H) were almost exclusively found in the small intestinal GISTs. Paraganglioma-like histologic features were observed in five cases of small intestinal GISTs as well as 8 gastric GISTs of the mixed spindle and epithelioid histologic types (Fig. 5I). These paraganglioma-like features were apparent in the superficial areas of the mass that possessed plump vascular structures.
Of the 747 GISTs, c-kit expression was found in 699 cases (93.6%) and its expression, according to the histologic diagnosis, for defining the risk of aggressive behavior is depicted in Fig. 6. c-kit was positive in 90.2% of very low risk, 94.0% of low risk, 93.7% of intermediate risk, and 95.3% of high risk GISTs (p>0.05). The c-kit expression was noted to be diffuse in the cytoplasm and along the cytoplasmic membrane of the tumor cells; in 24 cases, a dot-like c-kit expression was predominant rather than the membranous staining. Focal c-kit expression was observed in a total of 34 cases with cytoplasmic (29 cases) and a dot-like pattern (5 cases). In most of these cases, the pathologic diagnoses were very low and low risk of malignant potential and their histologic types were spindle cell type in 20 cases, epithelioid cell type in 5 cases, and mixed cell type in 5 cases.
CD34, SMA and S-100 protein were positive in 597 (80.2 %), 209 (28.1%), and 153 (20.5%) cases, respectively (Table 3). The GISTs with c-kit expression were related with CD34 positivity and with high cellularity of GISTs (p<0.05). But patients' gender, age, resection range, size of tumor, clinical stage, histologic type, presence of benign cellular pattern, necrosis and expression of S-100 protein were not related with the c-kit expression status (p>0.05). According to the locations of GISTs, c-kit positivity was high in the stomach (94.2%) and the small intestine (94.6%), while it was relatively low in the colorectum (85.0%), and the esophagus (81.2%). Additionally, all three cases with lymph node metastasis showed diffuse c-kit staining.
When dividing the GISTs according to their differentiation based on the immunophenotypic features, GIFT was the most common type (59.4%), followed by GILT (20.2%), GINT (12.5%), and GIDT (7.9%), respectively (Table 4). Fibroblastic differentiation (GIFT) was most frequent in the highly cellular and epithelioid GISTs, and it was more prevalent in the stomach and colorectum. Neural differentiation (GINT) was common in the small intestine, while smooth muscle differentiation (GILT) was common in the esophagus (Table 4).
Although several papers describing the clinicopathologic characteristics (20-22, 25, 26), the genetic (23, 24), and ultrastructural findings (27) of GISTs in Koreans have been reported, this multi-institutional study provides much more information on the clinical and immunophenotypic characteristics of GISTs in Koreans. In this study, we found that small intestinal GISTs were more common while colorectal and esophageal GISTs were less frequent in Koreans than in the Western countries (9, 17, 18). Like the previous reports suggesting the aggressive behavior of small intestinal GISTs, our survival data showed that the small intestinal GISTs comprised the most common cause of death by GISTs, although they made up only 30% of the total GISTs.
It is known that the epithelioid GISTs, which are the same as leiomyoblastomas of Stout, comprise about 10% of the gastric GISTs (9). In our series, 50 out of the 470 gastric GISTs (10.6%) were of epithelioid type. The spindle cell type was more frequent than epithelioid type and these results are in good agreement with the previous data (1, 5, 17).
In immunohistochemistry, c-kit was positive in 81-100% of the GISTs arising in the esophagus, stomach, small intestine and colon. The GISTs with either spindle cell or epithelioid type were both positive for c-kit, though the staining was less intense in the latter. The predominant membranous staining that can be observed in fibroblasts or myofibroblasts with the rabbit polyclonal antibody manufactured by Dako (28) was not observed in any of the cases we examined. CD34 was positive in 47-100% of the GISTs and its expression varied with the location of the tumor within the gastrointestinal tract. Miettinen et al. (10) found that among the c-kit positive tumors that they studied, 100%, 90%, 47%, 65%, 96% and 64% of the cases were CD34 positive in the esophagus, stomach, small intestine, colon, rectum and extraintestinal locations, respectively. In this study, CD34 expressions were 90%, 78%, 63% and 62% of the cases in the stomach, colorectum, small intestine and esophagus, respectively. Although CD34 positivity in the stomach was the same as that of previous study, its expression was somewhat different in the esophagus and small intestine. There have been some reports that CD34 is, perhaps, more often negative in malignant GISTs (29), although the opposite was found by Wang et al. (30). In our study, CD34 expression was positively correlated with the higher risk of malignancy in GISTs and this result may have been caused by higher positivity of CD34 in the small intestine and the lower CD34 expression in the esophagus. SMA was found only focally in 0-47% of the total cases and its expression rate was inversely correlated to that of CD34 (15-19). In our study, SMA was positive in 28.1% of the cases and it was most frequently expressed in the esophageal GISTs. Moreover, SMA expression was inversely correlated with the higher risk of malignancy in GISTs. However, the expression of S-100 protein in this study was higher than in the previous western studies, which were reported to be less than 10% of the cases with S-100 protein expression (1, 5, 17), and our findings were correlated well with the previous Korean reports (20). In the previous reports, the S-100 protein expression in GISTs was different according to the location of the tumor; it was more frequently expressed in the small intestinal GISTs (12, 31), and it was rarely expressed in the large intestine (11). The possible reasons for this high positivity may have been the higher incidence of small intestinal GISTs (20% vs. 30%), the lower incidence of colorectal GISTs (10% vs. 5%), the higher percentage of multiple and malignant GISTs in Koreans, and the differences in the interpretation of the results.
With this study, we found that expressions of CD34 and SMA were positively and inversely correlated with the higher risk of malignant potential in GISTs. The focal positivity of c-kit was associated with lower risk of malignant potential. According to the immunophenotypic features, GIFTs were common in the stomach and the colon. GILTs were common in the esophagus and GINTs were common in the small intestine. The higher incidence of GISTs with high risk of malignant potential and the more frequent small intestinal GISTs in Korean resulted in a higher death rate.
For further study, we need to continue with a) following up on these patients and completing the clinical data on recurrence, metastasis and survival, b) detecting the histologic and immunohistochemical markers that correlate with the prognosis, and c) performing genetic studies of kit and PDGFRA using kit-negative and multiple GISTs.
Figures and Tables
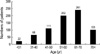 | Fig. 1Distribution of the ages of the patients with gastrointestinal stromal tumors in 747 patients. |
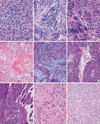 | Fig. 5Photograph of the representative findings of gastrointestinal stromal tumors. (A) Epithelioid type GIST. (B) Spindle cell type GIST. (C) Mixed epithelioid and spindle cell type GIST. (D) hyaline changes observed in GIST. (E) Myxoid changed observes in GIST. (F) Ischemic tumor necrosis observed in GIST. (G) Mucosal invasion observed in the small intestinal mucosa. (H) Skeinoid fibers observed in the small intestinal GIST. (I) Paraganglioma-like patterns observed in the small intestinal GIST. |
 | Fig. 6c-kit expression according to the pathologic diagnosis defining the risk of aggressive behavior for the gastrointestinal stromal tumors. |
Appendix
APPENDIX
Hospitals that participated in this study
Seoul National University Hospital, Chungnam National University Hospital, Yonsei University Hospital, Yong-Dong Severance Hospital, Yonsei Wonju University Hospital, Korea University Anam Hospital, Korea University Guro Hospital, Korea University Ansan Hospital, University of Ulsan Asan Medical Center, Sungkyunkwan University Samsung Seoul Hospital, Sungkyunkwan University Kangbuk Samsung Hospital, The Catholic University of Korea Kangnam St. Mary's Hospital, The Catholic University of Korea St. Mary's Hospital, The Catholic University of Korea St. Paul's Hospital, The Catholic University of Korea Our Lady of Mercy Hospital, The Catholic University of Korea St. Vincent's Hospital, The Catholic University of Korea Holy Family Hospital, The Catholic University of Korea Uijongbu St. Mary's Hospital, The Catholic University of Korea Daejeon St. Mary's Hospital, Kyung Hee University Hospital, Soonchunhyang University Hospital, Soonchunhyang University Chunan Hospital, Soonchunhyang University Puchon Hospital, Inje University Seoul Paik Hospital, Inje University Ilsan Paik Hospital, Inje University Pusan Paik Hospital, Ajou University Hospital, Eulji University Hospital, Nowon Eulji General Hospital, National Cancer Center Hospital, Korea Cancer Center Hospital, Seoul Municipal Boramae Hospital, Pundang Jesaeng Hospital, Pusan National University Hospital, Dong-A University Hospital, Kosin University Hospital, Pusan Maryknoll Hospital, Pusan Wallace Memorial Baptist Hospital, Kyungpook National University Hospital, Keimyung University Hospital, Yeungnam University Hospital, Daegu Catholic University Hospital, Taegu Fatima Hospital, Chosun University Hospital, Chonbuk National University Hospital, Wonkwang University Hospital, Chonju Prebyterian Medical Center, Konyang University Hospital, Chungbuk National University Hospital, Cheongju St. Mary's Hospital.
References
1. Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol. 2003. 54:3–24.
2. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004. 22:3813–3825.

3. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998. 279:577–580.

4. Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999. 154:53–60.

5. Miettinen MM, Sarlomo-Rikala M, Kovatich AJ, Lasota J. Calponin and h-caldesmon in soft tissue tumors: consistent h-caldesmon iommunoreactivity in gastrointestinal tumors indicates traits of smooth muscle differeutiation. Mod Pathol. 1999. 12:756–762.
6. Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998. 152:1259–1269.
7. Sakurai S, Fukasawa T, Chong JM, Tanaka A, Fukayama M. Embryonic form of smooth muscle myosin heavy chain (SMemb/MHC-B) in gastrointestinal stromal tumor and interstitial cells of Cajal. Am J Pathol. 1999. 154:23–28.

8. Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA, Demetri GD, Fletcher CD, Fletcher JA. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001. 61:8118–8121.
9. Miettinen M, Lasota J. Gastrointestinal stromal tumors; definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001. 438:1–12.
10. Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999. 30:1213–1220.

11. Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001. 25:1121–1133.
12. Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003. 27:625–641.

13. Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003. 299:708–710.

14. Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003. 125:660–667.
15. Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumours (GISTs): a review. Eur J Cancer. 2002. 38:Suppl 5. 39–51.
16. Rudolph P, Chiaravalli AM, Pauser U, Oschlies I, Hillemanns M, Gobbo M, Marichal M, Eusebi V, Hofler H, Capella C, Kloppel G. Gastrointestinal mesenchymal tumors-immunophenotypic classification and survival analysis. Virchows Arch. 2002. 441:238–248.
17. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002. 33:459–465.

18. Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999. 23:82–87.
19. O'Leary T, Berman JJ. Gastrointestinal stromal tumors: answers and questions. Hum Pathol. 2002. 33:456–458.
20. Kim MK, Lee JK, Park ET, Lee SH, Seol SY, Chung JM, Kang MS, Yoon HK. Gastrointestinal stromal tumors: clinical, pathologic features and effectiveness of new diagnostic criteria. Korean J Gastroenterol. 2004. 43:341–348.
21. Lee EJ, Lee OJ, Kim TH, Jung WT. A clinical and immunohistochemical study on gastrointestinal stromal tumor. Korean J Gastroenterol. 2003. 42:204–211.
22. Kim TW, Lee H, Kang YK, Choe MS, Ryu MH, Chang HM, Kim JS, Yook JH, Kim BS, Lee JS. Prognostic significance of c-kit mutation in localized gastrointestinal stromal tumors. Clin Cancer Res. 2004. 10:3076–3081.

24. Kang DW, Kim JH, Kim DH, Kim KH, Park MJ, Kang DY. c-kit mutation and immunohistochemical expression in gastrointestinal stromal tumors. Korean J Pathol. 2003. 37:246–254.
25. Ryu JS, Lee SR, Choi SB, Park SS, Lee JH, Kim SJ, Kim CS, Chae YS, Mok YJ. Gastrointestinal stromal tumor (GIST) of the stomach: clinicopathologic analysis and outcome? J Korean Gastric Cancer Assoc. 2005. 5:40–46.

26. Kwon SJ. Korean Gastric Cancer Study Group. Surgery and prognostic factors for gastric stromal tumor. World J Surg. 2001. 25:290–295.

27. Park SH, Kim MK, Kim HS, Song BJ, Chi JG. Ultrastructyral studies of Gastrointestinal stromal tumors. J Korean Med Sci. 2004. 19:234–244.
28. Yantiss RK, Spiro IJ, Compton CC, Rosenberg AE. Gastrointestinal stromal tumor versus intra-abdominal fibromatosis of the bowel wall. Am J Surg Pathol. 2000. 24:947–957.

29. Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999. 23:377–389.

30. Wang L, Vargas H, French SW. Cellular origin of gastrointestinal stromal tumors: a study of 27 cases. Arch Pathol Lab Med. 2000. 124:1471–1475.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download