Abstract
To verify the spectrum of CD99-expressing lymphoid malignancy, an immunohistochemical study for CD99 was carried out in 182 cases of non-Hodgkin's lymphoma, including 21 lymphoblastic lymphomas, 11 small lymphocytic lymphomas, 9 mantle cell lymphomas, 12 follicular lymphomas, 37 diffuse large B cell lymphomas, 18 Burkitt's lymphomas, 28 NK/T-cell lymphomas, 8 angioimmunoblastic T-cell lymphomas, 23 peripheral T-cell lymphomas, unspecified, and 15 systemic anaplastic large cell lymphomas. CD99 was positive in all T-lymphoblastic lymphomas and in 60% of B-lymphoblastic lymphomas. Majority of T and NK cell lymphomas were negative for CD99, except anaplastic large cell lymphomas (ALCLs). Eight of 15 cases (54%) of ALCLs reacted with anti CD99 antibody. Seven of 10 (70%) ALK positive ALCLs expressed CD99, whereas only 1 of 5 (20%) ALK negative ALCLs were positive. Of the mature B-cell lymphomas, 5.4% (2/37) of diffuse large B cell lymphomas and 11.1% (2/18) of Burkitt's lymphomas expressed CD99. In conclusion, CD99 is infrequently expressed in mature B and T cell lymphomas, except ALK-positive ALCL. High expression of CD99 in ALK-positive ALCL is unexpected finding and its biologic and clinical significances have yet to be clarified.
CD99 is a 32-kDa cell surface sialoglycoprotein, which is expressed in a wide variety of human cells. Functionally, CD99 is considered to act as an adhesion molecule or as a signal-transducing molecule (1, 2). Since original report that demonstrated its diagnostic utility in Ewing's sarcoma/primitive neuroectodermal tumor (3), CD99 expression has been demonstrated in a broad range of normal and neoplastic cells (4-10). In hematologic neoplasms, CD99 expression is well correlated with TdT positivity (11) and has been observed in T- and B-lymphoblastic lymphomas/leukemias, 55% of chloromas, 43% of acute myelogenous leukemias, and chronic myelogenous leukemias in blastic crisis (12). While CD99 is considered a useful marker for detecting lymphoblastic neoplasms, previous studies on mature T- and B cell non-Hodgkin's lymphomas (NHLs) have reported discordant results concerning the spectrum of CD99-positivity (11, 13, 14). In this study we examined CD99 immunoreactivity in a variety of NHL, including 21 lymphoblastic lymphomas/leukemias, 87 mature B-cell lymphomas, and 74 mature T and NK cell lymphomas.
During the period from January 1996 to April 2004, 182 patients diagnosed as having non-Hodgkin's lymphoma were randomly selected at Samsung Medical Center, Seoul, Korea. The selected cases comprised 21 lymphoblastic lymphomas/leukemias, 37 diffuse large B cell lymphomas, 18 Burkitt's lymphomas, 12 follicular lymphomas, 11 small lymphocytic lymphomas, 9 mantle cell lymphomas, 28 NK/T-cell lymphomas, 8 angioimmunoblastic T-cell lymphomas, 23 peripheral T-cell lymphomas, and 15 anaplastic large cell lymphomas. Clinical and laboratory records including a chromosomal study were reviewed.
Hematoxylin eosin-stained slides were reviewed in all cases and the diagnosis was made based on recent WHO classification (15).
Paraffin sections were stained with monoclonal CD99 antibody, 12E7 (1:40, DAKO, Carpinteria, CA, U.S.A.) in an automated staining system. Antigen retrieval was conducted in a microwave oven in citrate buffer at pH 6.0 before incubation with primary antibody. The tumors were considered immunoreactive for CD99 if diffuse cell membrane staining was observed in more than 70% of tumor cells. Focal or weak staining was interpreted as negative. In addition, paraffin sections were stained with ALK1 monoclonal antibody at a dilution of 1:40 (DAKO, Glostrup, Denmark), polyclonal antibody for CD3 (1:200, Novocastra, U.K.), monoclonal antibody for CD20 (1:500, Novocastra), monoclonal antibody for bcl-2 (1:40, DAKO), polyclonal antibody for bcl-6 (1:40, Santacruz, California, U.S.A.), monoclonal antibody for CD10 (1:50, Novocastra, U.K.), monoclonal antibody for CD56 (1:20, Monosan, New Zealand), monoclonal antibody for CD30 (1:30, DAKO, Glostrup, Denmark), and with monoclonal antibody for CD21 (1:25, DAKO, Glostrup, Denmark).
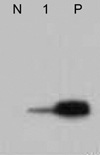
To verify CD99 expression in anaplastic large cell lymphoma, western blot analysis was performed in one case of anaplastic large cell lymphoma of which fresh tumor tissue was available. For the positive control, lymphoblastic lymphoma was used. Monoclonal CD99 antibody, 12E7 (DAKO, Carpinteria, CA) was applied at a dilution of 1:1,000.
The results of immunohistochemical staining for CD99 are summarized in Table 1.
CD99 was positive in 100% of T-lymphoblastic lymphomas, but in only 60% of B-lymphoblastic lymphomas/leukemias. The majority of mature T and NK cell lymphomas were negative for CD99, except anaplastic large cell lymphomas. Fifty-four percent (8/15) of systemic ALCLs reacted with anti CD99 antibody. ALK positive tumors expressed CD99 more frequently than ALK negative tumors; 7 of 10 (70%) ALK positive ALCLs expressed CD99 (Fig. 1), whereas only 1 of 5 (20%) ALK negative ALCLs were positive. The intensity of CD99 in ALCL was rather weaker than that of lymphoblastic lymphoma, but cytoplasmic membrane stain of tumor cells was obvious which was in accord with the result of western blot analysis (Fig. 2). Of the mature B cell lymphomas, 5.3% (2/37) of diffuse large B cell lymphomas and 11.1% (2/18) of Burkitt's lymphomas were positive for CD 99. All follicular lymphomas, small lymphocytic lymphomas, and mantle cell lymphomas were negative for CD99.
Of the 2 cases of CD99-positive diffuse large B cell lymphoma, one was a 51-yr-old man who presented with axillary lymph node enlargement. This tumor was composed of large cells, which were positive for CD20, bcl-6, MUM-1, and EBV ISH, and negative for CD3 and CD10. The second patient was a 56-yr-old female who presented with an enlarged neck lymph node; tumor cells were large, had a diffuse appearance, and were positive for CD20 and bcl-6, and negative for CD10, bcl-2, MUM-1, and EBV ISH.
In the two CD99-positive cases of Burkitt's lymphomas, one patient was a 78-yr-old man who present with a stomach lesion. Tumor cells showed strong bcl-6 and CD20 expression, but were negative for bcl-2, and had a Ki-67 labeling index more than 99%. The other patient was 65-yr-old man with a retroperitoneal tumor, which was negative for TdT and positive for CD79a and CD20 with a Ki-67 labeling index of 90%. In this case bone marrow metastasis was identified at the initial diagnosis and a chromosomal study revealed t(8:14). Microscopically tumor cells were monotonous with round to oval nuclei and a prominent nuclear membrane. Nucleoli were prominent and ranged from 2 to 5 per nucleus. Occasional tumor cells had a centrally located single prominent nucleolus resembling an immunoblast. The chromatin was coarse and irregularly distributed.
CD99 is a 32-kDa transmembrane sialoglycoprotein that is expressed on many types of human cells. However, despite its broad distribution CD99 is only strongly expressed in relatively a few cell types, such as bone marrow progenitor cells, pancreatic islet cells, granulosa cells of the ovary, and Leydig and Sertoli cells of the testis and ependyma (3, 7, 16). CD99 was originally described as a diagnostically useful marker for Ewing's sarcoma/primitive neuroectodermal tumor (3), but CD99 immunoreactivity has also been documented in a variety of other tumors, including sex cord tumors of the testis and ovary, synovial sarcoma, chondrosarcoma, and neuroendocrine tumors (6-8). Moreover, within the hematopoietic system CD99 expression is strongly correlated with TdT expression, in addition it is highly expressed in early CD34+ precursors, which is in line with the maturation process (16, 17). In hematologic neoplasms, CD99 expression has been observed in T-and B-lymphoblastic lymphomas/leukemias, chloroma, acute myelogeneous leukemia, and chronic myelogeneous leukemia in blastic crisis (12, 13).
The expression of CD99 in non-Hodgkin's lymphomas, other than those of the lymphoblastic type has become the subject of debate. One study had reported a negative reaction in all NHLs, other than lymphoblastic lymphoma (9), whilst the other had described only weak, non-uniform, poorly localized cytoplasmic staining in a minority of cases (13). Vartanian et al. (14), using a heat-induced epitope retrieval (HIER) technique, reported that a variety of malignant lymphomas, other than the lymphoblastic type, show significant CD99 reactivity. However, Robertson et al. (11), also using HIER, found that positive CD99 staining was restricted to lymphoblastic disorders, and ascribed this discrepancy to technical differences. The present study, also using HIER, revealed distinct membranous CD99 staining in a few cases of NHL of the mature phenotype, and unexpectedly high expression in ALK-positive ALCLs. Twenty percent of ALK-negative ALCLs expressed CD99, and this rose to 70% in ALK-positive ALCLs.
How the high expression of CD99 in ALK positive ALCLs be explained? Though there appears to be no clear-cut answer to this question, present data suggest that ALK protein may play a role. CD30 is a member of the tumor necrosis factor receptor (TNFR) superfamily and one of essential findings for the diagnosis of ALCL and Hodgkin's disease (18, 19). In Hodgkin's disease (HD), constitutively activated NF-κB by Hodgkin's disease (HD), constitutively activated NF-κB by either EBV LMP-1 gene or CD30, down-regulates the expression of CD99 and the repression is restored by the inhibition of NF-κB activity (20). In ALK-positive ALCL, NF-κB activation is not observed in the presence of equivalent levels of CD30 expression (21) because NPM-ALK oncoprotein abrogates CD30 signaling and constitutive NF-κB activation (22).
The functional roles of CD99 expressed in ALCL are unknown. The CD99 signal enhances Fas-mediated apoptosis in the human leukemic cell line (23). Engagement of distinct epitopes on CD99 rapidly induces T cell death by a novel caspase-independent pathway through reorganization of cytoskeleton (24). Because ALK-positive ALCLs show higher apoptosis rate and higher levels of active procaspase 3 and proapoptotic Bcl-2 family proteins such as BAX and BCL-XS than ALK-negative ALCLs, it is conceivable that CD99 works on apoptotic cell death in ALK-positive ALCLs (25, 26).
In summary, present study demonstrates that CD99 expressed frequently in ALK-positive ALCL, but not in other mature T and NK cell lymphoma. The result expands our understanding on the spectrum of CD99-positive neoplasm although its biologic and clinical significance remained to be clarified.
Figures and Tables
 | Fig. 1(A) Anaplastic large cell lymphoma. H & E stain (×400). (B) Anaplastic large cell lymphoma, CD30 expression (×100). (C) Anaplstic large cell lymphoma, ALK protein (×100). (D) Anaplastic large cell lymphoma, CD99 expression (×100). (E) Anaplastic large cell lymphoma. Higher magnification of CD99 immunostain (×400). |
References
1. Hahn JH, Kim MK, Choi EY, Kim SH, Sohn HW, Ham DI, Chung DH, Kim TJ, Lee WJ, Park CK, Ree HJ, Park SH. CD99 (MIC2) regulates the LFA-1/ICAM-1-mediated adhesion of lymphocytes, and its gene encodes both positive and negative regulators of cellular adhesion. J Immunol. 1997. 159:2250–2258.
2. Waclavicek M, Majdic O, Stulnig T, Berger M, Sunder-Plassmann R, Zlabinger GJ, Baumruker T, Stockl J, Ebner C, Knapp W, Pickl WF. CD99 engagement on human peripheral blood T cells results in TCR/CD3-dependent cellular activation and allows for Th1-restricted cytokine production. J Immunol. 1998. 161:4671–4678.
3. Hamilton G, Fellinger EJ, Schratter I, Fritsch A. Characterization of a human endocrine tissue and tumor-associated Ewing's sarcoma antigen. Cancer Res. 1988. 48:6127–6131.
4. Weidner N, Tjoe J. Immunohistochemical profile of monoclonal antibody O13: antibody that recognizes glycoprotein p30/32MIC2 and is useful in diagnosing Ewing's sarcoma and peripheral neuroepithelioma. Am J Surg Pathol. 1994. 18:486–494.
5. Ramani P, Rampling D, Link M. Immunocytochemical study of 12E7 in small round-cell tumours of childhood: an assessment of its sensitivity and specificity. Histopathology. 1993. 23:557–561.

6. Pelosi G, Fraggetta F, Sonzogni A, Fazio N, Cavallon A, Viale G. CD99 immunoreactivity in gastrointestinal and pulmonary neuroendocrine tumours. Virchows Arch. 2000. 437:270–274.

7. Gordon MD, Corless C, Renshaw AA, Beckstead J. CD99, keratin, and vimentin staining of sex cord-stromal tumors, normal ovary, and testis. Mod Pathol. 1998. 11:769–773.
8. Granter SR, Renshaw AA, Fletcher CD, Bhan AK, Rosenberg AE. CD99 reactivity in mesenchymal chondrosarcoma. Hum Pathol. 1996. 27:1273–1276.

9. Jung KC, Park WS, Bae YM, Hahn JH, Hahn K, Lee H, Lee HW, Koo HJ, Shin HJ, Shin HS, Park YE, Park SH. Immunoreactivity of CD99 in stomach cancer. J Korean Med Sci. 2002. 17:483–489.

10. Yoo SH, Han J, Kim TJ, Chung DH. Expression of CD99 in pleomorphic carcinomas of the lung. J Korean Med Sci. 2005. 20:50–55.

11. Robertson PB, Neiman RS, Worapongpaiboon S, John K, Orazi A. 013 (CD99) positive in hematologic proliferations correlates with TdT positivity. Mod Pathol. 1997. 10:277–282.
12. Zhang PJ, Barcos M, Stewart CC, Block AW, Sait S, Brooks JJ. Immunoreactivity of MIC2 (CD99) in acute myelogenous leukemia and related diseases. Mod Pathol. 2000. 13:452–458.

13. Riopel M, Dickman PS, Link MP, Perlman EJ. MIC2 analysis in pediatric lymphomas and leukemias. Hum Pathol. 1994. 25:396–399.

14. Vartanian RK, Sudilovsky D, Weidner N. Immunostaining of monoclonal antibody 013 [anti-MIC2 gene product (CD99)] in lymphomas; impact of heat-induced epitope retrieval. Appl Immunohistochem. 1996. 4:43–55.
15. Jaffe ES, Harris NL, Stein H, Vardiman J, editors. World Health Organization Classification of Tumors. Pathology & Genetics. Tumors of Hematopoietic and Lymphoid Tissues. 2001. Lyon: IARC Press.
16. Dworzak MN, Fritsch G, Buchinger P, Fleischer C, Printz D, Zellner A, Schollhammer A, Steiner G, Ambros PF, Gadner H. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood. 1994. 83:415–425.

17. Dworzak MN, Fritsch G, Fleischer C, Printz D, Froschl G, Buchinger P, Mann G, Gadner H. CD99 (MIC2) expression in paediatric B-lineage leukaemia/lymphoma reflects maturation-associated patterns of normal B-lymphopoiesis. Br J Haematol. 1999. 105:690–695.

18. Stein H, Herbst H, Anagnostopoulos I, Niedobitek G, Dallenbach F, Kratzsch HC. The nature of Hodgkin and Reed-Sternberg cells, their association with EBV, and their relationship to anaplastic large-cell lymphoma. Ann Oncol. 1991. Suppl 2. 33–38.

19. Leoncini L, Del Vecchio MT, Kraft R, Megha T, Barbini P, Cevenini G, Poggi S, Pileri S, Tosi P, Cottier H. Hodgkin's disease and CD30-positive anaplastic large cell lymphomas-a continuous spectrum of malignant disorders. A quantitative morphometric and immunohistologic study. Am J Pathol. 1990. 137:1047–1057.
20. Lee IS, Shin YK, Chung DH, Park SH. LMP1-induced down-regulation of CD99 molecules in Hodgkin and Reed-Sternberg cells. Leuk Lymphoma. 2001. 42:587–594.

21. Bargou RC, Leng C, Krappmann D, Emmerich F, Mapara MY, Bommert K, Royer HD, Scheidereit C, Dorken B. High-level nuclear NF-kappa B and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood. 1996. 87:4340–4347.

22. Horie R, Watanabe M, Ishida T, Koiwa T, Aizawa S, Itoh K, Higashihara M, Kadin ME, Watanabe T. The NPM-ALK oncoprotein abrogates CD30 signaling and constitutive NF-kappaB activation in anaplastic large cell lymphoma. Cancer Cell. 2004. 5:353–364.
23. Jung KC, Kim NH, Park WS, Park SH, Bae Y. The CD99 signal enhances Fas-mediated apoptosis in the human leukemic cell line, Jurkat. FEBS Lett. 2003. 554:478–484.

24. Cerisano V, Aalto Y, Perdichizzi S, Bernard G, Manara MC, Benini S, Cenacchi G, Preda P, Lattanzi G, Nagy B, Knuutila S, Colombo MP, Bernard A, Picci P, Scotlandi K. Molecular mechanisms of CD99-induced caspase-independent cell death and cell-cell adhesion in Ewing's sarcoma cells: actin and zyxin as key intracellular mediators. Oncogene. 2004. 23:5664–5674.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download