Abstract
The potential therapeutic benefit of introducing IFN-γ and GM-CSF genes in combination with the HSVtk suicide gene into subcutaneously implanted CT26 tumor cells was compared with that from each treatment alone. Cells, unmodified or retrovirally transduced with HSVtk or IFN-γ/GM-CSF genes, were inoculated subcutaneously into syngeneic BALB/c mice in various combinations. HSVtk gene, with intraperitoneal ganciclovir treatment, reduced tumor volume by 81% at locally inoculated tumor sites (p<0.01) and by 25% at distantly inoculated tumor sites (p=0.052). IFN-γ/GM-CSF genes showed a 56% tumor volume reduction at local tumor sites (p<0.01) and 15% volume reduction at remote tumor sites, although this was not statistically significant. The combination of HSVtk (with GCV) and IFN-γ/GM-CSF genes showed an 81% volume reduction at local tumor sites (p<0.01) and a 43% volume reduction at remote tumor sites (p<0.01). Thus, the combination of HSVtk and IFN-γ/GM-CSF gene therapy produced greater therapeutic efficacy than either treatment alone.
Popular strategies of cancer gene therapy have been to augment suicidal activity by introducing the Herpes simplex virus thymidine kinase (HSVtk) gene through ganciclovir (GCV) treatment (1-4) or to increase anti-tumor immune response by transfer of some cytokine genes such as IL-2 (5, 6), IL-4 (7), IFN-γ (8), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (9). These studies have yielded encouraging results, which have inspired investigators to optimize the combinations of suicide gene with cytokine genes or those of different cytokine genes to increase the anti-tumor effect.
The HSVtk converts nucleoside analogues, such as GCV, to monophosphate forms by phosphorylation. These are subsequently modified to toxic triphosphates by endogenous cellular enzymes and incorporated into nascent DNA, which causes chain termination and cell death. With such an alteration of GCV, neighboring cells that do not express the HSVtk gene are killed in addition to the tumor cells expressing the genes. As the phosphorylated GCV is unable to diffuse freely across the plasma membrane, several mechanisms have been suggested for this bystander killing effect. One of them is the connexin gap junction-mediated intercellular transfer of toxic phosphorylated GCV molecules (10, 11), although explanation is not always applicable to different tumor types (12). Some investigators have also reported that the bystander effect is impaired, or even abrogated, when experiments are conducted in immune compromised animals, which suggests that an intact immune system is an important component for obtaining a bystander effect (13). Within partly HSVtk positive tumors, GCV treatment induces local leucocytes infiltration and cytokine release (13, 14).
Anti-tumor immune response is believed to be largely dependent on major histocompatibility complex (MHC) class I-restricted CD8+, cytolytic T-cells. Interferon-γ (IFN-γ) is a pleiotropic cytokine produced by activated T-lymphocytes. It influences the immune response in several ways, largely because it is a potent inducer of MHC class I antigens, which may increase the antigen-presenting capacity of cells (15). GM-CSF induces proliferation and differentiation of cells committed to the granulocyte-macrophage lineage. It augments the antigen-presenting activity of macrophages and activates dendritic cells (16). In addition, multiple cytokine gene transfer showed additional or synergistic anti-tumor effects but the tumor growth was not completely inhibited (17).
In clinical situation, cancer gene therapy will be first applied to disseminated diseases or large bulky tumors that are refractory to current treatment modalities. However, current vector technologies do not enable transfer the therapeutic genes into sufficient number of malignant cells to produce curative effects within a solid tumor. Promise of viable treatment is suggested by the numerous experimental suicide gene therapies demonstrating a bystander killing effect of tumor cells. As we have already demonstrated the effects of HSVtk and GM-CSF combination gene therapy in a murine CT26 cancer cell models (18), multiple gene transfer is now becoming an important strategy in cancer gene therapy. In this study, examination was done whether combined HSVtk and IFN-γ/GM-CSF gene therapy could show enhanced anti-tumor effects over each treatment alone.
Six- to eight-week-old male syngeneic BALB/c mice from which CT26 cells, an N-nitroso-N-methylurethane (NNMU)-induced undifferentiated colon carcinoma line, originated were purchased from a commercial vender (SLC Inc, Japan). All animal protocols were performed according to the guidelines of the Chungbuk National University animal facilities and NIH. Mice were allowed access to food and water ad libitum for the duration of the experiment.
CT26 cells, and PA317, PE501 and NIH 3T3 cells were routinely maintained with DMEM (high glucose; Gibco BRL, U.S.A.) containing 10% fetal bovine serum (heat inactivated), 100 U/mL of penicillin-streptomycin and 2 mM glutamine under a 5% CO2-humidified air atmosphere at 37℃.
All retroviral vectors were cloned using standard techniques. The HSVtk expressing LtkSP vector was cloned as previously described (18). The INF-γ expressing LγSN vector was constructed by cloning the INF-γ cDNA into the HpaI site of LXSN, provided by Professor William RA Osborne (Department of Pediatrics, University of Washington, Seattle, WA, U.S.A.). The GM-CSF expressing LNFGM vector was constructed by cloning the GM-CSF cDNA into the BamHI site of LNFX. The INF-γ and GM-CSF co-expressing LγFGMEN vector was constructed as follows. A 975-base pair DNA fragment encoding FGM portion of LNFGM vector was obtained by digestion with restriction enzyme ClaI and fused with a unique site of LAEN to construct LAFGMEN. Then the LA part of LAFGMEN was replaced by the Lγ part of LγSN by digestion with the restriction enzymes SacII and BamHI to clone the final construct LγFGMEN.
The methods used to generate retroviral packaging cell lines minimizing the formation of replication-competent retrovirus, and to produce high-titer amphotropic vectors involving selection, dilution cloning, and screening, were as previously described (19).
The parental CT26 cells were transduced by each retrovirus using amphotropic retroviral packaging cell line (PA317) as previously described (19). CT26/TK cells were established by the addition of 1 µg/mL of puromycin for LtkSP, and CT26/γIFN, CT26/GM and CT26/γIFN-GM were also established by selection with 1 mg/mL G418 for LγSN, LNFGM and LγFGMEN, respectively. Cells were subcultured and grown thereafter in appropriate selection containing media.
Supernatants from 2×106 semiconfluent cells in a 6 cm plate of CT26/γIFN, CT26/GM and CT26/γIFN-GM were collected after 48 hr and assayed for mouse IFN-γ and GM-CSF using an ELISA kit (Endogen, Boston, MA, U.S.A.).
Quantitative analysis of MHC expression on cell surface was performed using a fluorescently activated cell sorter (FACS; Epics XL-MCL, Coulter, Miami, FL, U.S.A.). Cells were scraped from tissue culture plates using a rubber policeman and incubated with murine anti-H-2KdDd monoclonal antibody for 30 min at 4℃. After being washed, the cells were incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG antibodies at 4℃ for 30 min, fixed with 1% paraformaldehyde, and examined within one week using flow cytometry. In all cases controls incubated with secondary antibody alone failed to show any significant nonspecific binding.
Four groups of mice (n=15 to 20), comprising GCV (Cymevene, Roche products, Basel, Switzerland) treated or non-treated, were subcutaneously implanted with tumor cells on both flanks. The proportions of the cells implanted are listed in Table 1. The mixture of CT26/TK and unmodified CT26 (each 5×105 cells of each in 100 µL of phosphate-buffered saline) was implanted on the left flank and unmodified CT26 cells (5×105 cells) on the right flank as a control group (without GCV) and suicidal gene therapy group (with GCV). In cytokine (without GCV) and the combined gene therapy group (with GCV), the mixture of CT26/TK and CT26/γIFN-GM (each 5×105 cells) was implanted on the left flank and unmodified CT26 cells (5×105 cells) on the right flank. The tumors were left to develop for 4 days before the mice in each treatment group were intraperitoneally injected twice daily with GCV at a dose of 50 mg/kg for 14 days (from D5 to D18). The size of each tumor was measured using calipers and tumor volumes were calculated as: (longest diameter)× (shortest diameter)2/2 (20).
The significance of difference between groups was tested by a one-way ANOVA with the use of StatView 5.0 software (SAS Institute Inc., Cary. NC, U.S.A.). If a probability value of p<0.05 was obtained, the Tukey test was then used to compare each individual group with its appropriate control.
The concentrations of INF-γ from supernatants of CT26/γIFN and CT26/γIFN-GM cell lines were 630±34 and 716±65 pg/106 cells per day, respectively. There were no significant differences in production rate of INF-γ in either cell lines. The GM-CSF concentrations from CT26/GM and CT26/γIFN-GM cell lines were very similar (18.1±2.1 vs. 16.3±1.5 pg/106 cells per day) (Table 2). This means that the tricistronic retroviral vector cloned to co-express IFN-γ and GM-CSF was working as well as single gene expression vectors.
Prior to transfection studies, we ascertained whether the CT26 cells would express MHC class I antigen in response to produced IFN-γ. Fig. 1 shows the expression of H-2KdDd in CT26 cells as assessed by FACS using a specific murine monoclonal antibody. The mean fluorescence intensities (MFI) of unmodified CT26 and CT26/GM were 25 and 22.7, whereas the MFI of CT26/γIFN and CT26/γIFN-GM were 41.1 and 45.5, respectively. This demonstrates an increase in MHC class I antigen expression in only the IFN-γ transduced cell lines.
We next determined if expressed IFN-γ or GM-CSF had a cytostatic effect on CT26 cells. Unmodified CT26, CT26/TK, CT26/γIFN-GM, 50% CT26+50% CT26/TK and 50% CT26+50% CT26/γIFN-GM (1×104 cells) were plated and cultured for 8 days. The final cell number of those cell lines were 6×106 for CT26, 5×106 for CT26/TK, 5.5×106 for CT26/γIFN-GM, 4.5×106 for 50% CT26+50% CT26/TK and 4.4×106 for 50% CT26+50% CT26/γIFN-GM. There were no differences in growth rate between the different CT26 cell lines (Fig. 2).
The tumor volumes at left flank of suicide group were significantly lower than those of control group at day 11 (544±196 µL vs. 1,100±872 µL, p<0.05), day 15 (792±522 µL vs. 2,624±1,667 µL, p<0.01) and day 18 (1,116±1,037 µL vs. 5,980±2,768 µL, p<0.01) (Fig. 3A). There was an 81% volume reduction at day 18.
The cytokine group at the left flank also showed significantly lower mean tumor volume from day 11 (615±304 µL, p<0.05; 1,042±441 µL at day 15, p<0.01 and 2,609±1,187 µL at day 18, p<0.01 compared with each of the control groups) (Fig. 3B) and a 56% volume reduction at day 18.
The combined group also showed significant tumor volume reductions (394±187 µL at day 11, p<0.01; 601±450 µL at day 15, p<0.01 and 1,134±675 µL at day 18, p<0.01 compared with each of control groups) (Fig. 3C) and an 81% volume reduction at day 18.
The unmodified cells at the right flank of the suicide group showed decreased tumor volume (25% volume reduction) compared with that of the control group at day 18, although the difference was not statistically significant (4,976±1,323 µL vs. 6,690±3,469 µL, p=0.052, Fig. 4A).
In the cytokine group, there was a 15% volume reduction at day 18 compared with that of the control group, but it was not statistically significant (5,637±1,964 µL vs. 6,690±3,469 µL, Fig. 4B).
The unmodified cells at the right flank of the combined group showed significantly decreased tumor volumes from day 15 compared with those of the control group (1,942±540 µL vs. 2,961±1,648 µL at day 15, p<0.05; 3,840±1,430 µL vs. 6,690±3,469 µL at day 18, p<0.01) (Fig. 4C) and a 43% volume reduction at day 18. This shows that the combination of suicidal and cytokine gene therapy has an additional remote anti-tumor effect even though each treatment alone had no significant remote effect. This means that a 25% volume reduction could result from the distant anti-tumor effect of HSV-TK and an additional 18% volume decrease could result from the effect of the cytokine gene therapy (Fig. 5).
Suicide gene therapy and cytokine gene therapy are promising approaches for cancer gene therapy (21-23). Various strategies using HSVtk suicide gene therapy combined with local delivery of cytokine genes have been explored in preclinical models to target systemic neoplastic disease. Gene therapies using various cytokine genes combined with HSVtk suicide gene have been shown to increase anti-tumor effects leading to prolongation of survival, partial protection against a subsequent tumor challenge, and infiltration of immune cells into the tumor (24-27).
This study was undertaken to determine if cytokine gene therapy using IFN-γ and GM-CSF in combination with HSVtk suicide gene therapy could increase anti-tumor effects at local and distant tumor sites. For this purpose, we cloned retroviral vector expressing HSVtk and tricistronic retroviral vector to co-express IFN-γ and GM-CSF. This tricistronic vector expressed both cytokines as well as single gene expression vectors (Table 2).
Rodent tumor cells have been engineered to secrete cytokines locally in an attempt to elicit immune responses against the tumors (28). Secretion of IL-2, IL-4, IFN-γ or GM-CSF by a variety of such tumor cells was shown to reduce the tumorigenicity of these cells in syngeneic animals (17, 29, 30). In this context, the genes for GM-CSF and IFN-γ have been studied most extensively to date. GM-CSF has potential as an important anti-neoplastic agent (16). Armstrong et al. (9) reported that GM-CSF expression in the weakly immunogenic murine B16-derived malignant melanoma cell line HFH18 reduced tumorigenicity and induced protective immunity in animals. In addition, they showed that GM-CSF could induce an anti-tumor effect by recruiting dendritic antigen-presenting cells. IFN-γ has also been reported to induce a potent anti-tumor immune response, largely because of the induction of MHC class I antigens. In this study, the FACS results show that the MHC class I antigen was increased in the IFN-γ-transduced cell lines. This result is compatible with the finding of Webber and Rosenberg (15).
In our study, HSVtk suicide gene therapy alone showed a significant local bystander effect, in which an 80% volume reduction was produced after inoculation of 50% CT26+50% CT26/TK at the left flank compared with the control group. This means that each HSVtk expressing cell could inhibit approximately 0.6 unmodified neighboring cells in vivo. This result is compatible with the finding of Lee et al. (18) that two HSVtk cells could kill one neighbouring unmodified cell in vivo. But, one HSVtk expressing cell in vitro could kill two adjacent cells. Suicide gene treatment alone also showed a 25% tumor volume reduction at distant tumor sites, although the p-value was 0.052. The mechanism for the distant bystander effect of HSVtk is not fully understood. Although the transfer of toxic metabolites via gap junction has a role, it is not possible to explain the distant anti-tumor effect. It is known that HSVtk has a distant bystander effect via an immunologic mechanism, in which the release of tumor antigens by dying cells may induce systemic anti-tumor immunity (31, 32).
Cytokine gene therapy alone also showed a local anti-tumor effect, in which a 56% volume reduction was produced, but this result was smaller than suicide gene treatment alone (56% vs. 81%). This means that cytokine gene therapy can evoke the local anti-tumor effect but it is weaker than suicidal gene therapy. The distant anti-tumor effect was not shown in our study design, which was to sacrifice animals at day 18. However, this finding is compatible with the report of Gansbacher et al. (33) that IFN-γ producing CMS-5 cells on the left flank did not inhibit the growth of unmodified CMS-5 cells on the opposite flank. This also explains why the effect of IFN-γ production by the tumor cells initially appeared to stimulate a local rather than a systemic immune response but ultimately led to a potent long-term protective immunity. Considering the short-term duration of this study, an additional study with a longer treatment period of cytokine gene therapy is needed to show a distant anti-tumor effect.
Finally, combined gene therapy showed significantly decreased local and distant tumor volumes, in which an 81% volume reduction at the locally inoculated tumor and a 43% volume reduction at distantly inoculated tumor were obtained. In addition, combined gene therapy showed earlier tumor reduction from day 15 than did suicide treatment alone. This means that combined gene therapy has an earlier and stronger anti-tumor effect compared with each treatment alone.
In summary, our study shows that cytokine gene therapy using IFN-γ and GM-CSF genes in combination with HSVtk suicide gene therapy could be an effective treatment modality for cancer gene therapy.
Figures and Tables
 | Fig. 1Expression of MHC class I molecules in untransduced or transduced CT26 cells. INF-γ transduced cells only showed increased expression. Cells were stained for FITC-labeled H-2 KdDd.
MFI, mean fluorescence intensity in region C.
|
 | Fig. 2In vitro growth of untransduced or transduced CT26 cells used in animal study. There is no difference of growth rate in different cell lines. |
 | Fig. 3Tumor volumes of transduced CT26 cells at locally inoculated tumor. (A) control vs. suicide gene therapy, (B) control vs. cytokine gene therapy, (C) control vs. combined gene therapy.
Differences were significant: *p<0.05, †p<0.01. Results were expressed as mean±SD.
|
 | Fig. 4Tumor volumes of untransduced CT26 cells at distantly inoculated tumor. (A) control vs. suicide gene therapy, (B) control vs. cytokine gene therapy, (C) control vs. combined gene therapy.
Differences were significant: *p=0.052, †p<0.05, ‡p<0.01. Results were expressed as mean±SD.
|
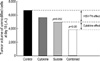 | Fig. 5The therapeutic effects of HSVtk and cytokines expression on tumor growth at distantly inoculated tumor. |
References
1. Dilber MS, Abedi MR, Bjorkstrand B, Christensson B, Gohrton G, Xanthogoulous KG, Smith CI. Suicide gene therapy for plasma cell tumors. Blood. 1996. 88:2192–2200.

2. Kim YG, Kim ST, Yoon HD. Enhanced in vitro and in vivo bystander effect by double transfer of herpes simplex virus thymidine kinase gene. J Korean Neurosurg Soc. 1999. 28:1407–1417.
3. Kianmanesh AR, Perrin H, Panis Y, Fabre M, Nagy HJ, Houssin D, Klatzmann D. A distant bystander effect of suicide gene therapy; Regression of nontransduced tumors together with a distant transduced tumor. Human Gene Ther. 1997. 8:1807–1814.

4. Agard C, Ligeza C, Dupas B, Izembart A, Kouri CE, Moullier P, Ferry N. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001. 8:128–136.

5. Fearson E, Hunt B, Itaya T, Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an anti-tumor response. Cell. 1990. 60:397–403.

6. Patel PM, Fleming CL, Russel SJ, McKat IA, MacLennan KA, Box GM, Eccles SA, Collins MK. Comparison of the potential therapeutic effect of interleukin 2 or interleukin 4 secretion by a single tumor. Brit J Cancer. 1993. 68:295–305.
7. Golumbek PT, Lazenby AJ, Levitsky HI, Jaffee LM, Karasuyama H, Baker M, Pardoll DM. Treatment of established renal cancer by tumor cells engineered to secrete IL-4. Science. 1991. 254:713–716.
8. Esumi N, Hunt B, Itaya T, Frost P. Reduced tumorigenicity of murine tumor cells secreting IFN-γ is due to nonspecific host responses and is unrelated to class I major histocompatibility complex expression. Cancer Res. 1991. 51:1185–1189.
9. Armstrong CA, Botella R, Galloway TH, Murray N, Kramp JM, Song IS, Ansel JC. Antitumour effects of granulocyte-macrophage colony-stimulating factor production by melanoma cells. Cancer Res. 1996. 56:2191–2198.
10. Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996. 93:1831–1835.

11. Burrows FJ, Gore M, Smiley WR, Kanemitsu MY, Jolly DJ, Read SB, Nicholas T, Kruse CA. Purified herpes simplex virus thymidine kinase retroviral particles; III. Characterization of bystander killing mechanisms in transfected tumor cells. Cancer Gene Ther. 2002. 9:87–95.
12. Beck C, Cayeux S, Lupton SD, Dorken B, Blankenstein T. The thymidine kinase/ganciclovir-mediated suicide effect is variable in different tumor cells. Hum Gene Ther. 1995. 6:1525–1530.

13. Freeman SM, Whartenby KA, Freeman JL, Abboud CN, Marrogi AJ. In situ use of suicide genes for cancer therapy. Semin Oncol. 1996. 23:31–45.
14. Caruso M, Paris Y, Gagandeep S, Houssin P, Saltzmann JL, Klatzmann D. Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci USA. 1993. 90:7024–7028.

15. Weber JS, Rosenberg SA. Modulation of murine tumor major histocompatibility antigens by cytokines in vitro and in vivo. Cancer Res. 1988. 48:5818–5824.
16. Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligar RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony stimulating factor stimulates potent, specific, and long lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993. 90:3539–3543.
17. Kim ST, Lee KH, Oh TG, Kim YG. Effect of combined gene therapy using tricistronic retroviral vector containing gamma-interferon and granulocyte-macrophage colony-stimulating factor into CT26 cells. Korean J BRM. 1999. 9:41–49.
18. Lee KH, Piao H, Son BR, Heo DS, Kim NK, Kim ST. Herpes simplex virus thymidine kinase and granulocyte macrophage colony-stimulating factor combination gene therapy in a murine CT26 cell colon cancer model. Cancer Gene Ther. 2004. 11:570–576.

19. Kruse CA, Roper MD, Kleinschmidt-DeMasters BK, Banuelos SJ, Smiley WR, Robbins JM, Burrows FJ. Purified herpes simplex thymidine kinase retrovector particles. I. In vitro characterization, in situ transduction efficiency, and histopathological analyses of gene therapy-treated brain tumors. Cancer Gene Ther. 1997. 4:118–128.
20. Rockwell SC, Kallimann RF, Fajardo LF. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst. 1972. 49:735–749.
21. Ezzedine ZD, Martuza RL, Plaitika D. Selective killing of glioma cells in culture and in vivo by retrovirus transfer of the herpes simplex virus thymidine kinase gene. New Biol. 1991. 3:608–614.
22. Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blease RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992. 256:1550–1552.

23. Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995. 13:399–415.

24. Chen SH, Kosai K, Xu B, Pham-Nguyen K, Contant C, Finegold MJ, Woo SL. Combination suicide and cytokine gene therapy for hepatic metastases of colon carcinoma: sustained antitumor immunity prolongs animal survival. Cancer Res. 1996. 56:3758–3762.
25. Bonnekoh B, Greenhalgh DA, Chen SH, Block A, Rich SS, Kreig T, Woo SL, Roop DR. Ex vivo and in vivo adenovirus-mediated gene therapy strategies induce a systemic anti-tumor immune defence in the B16 melanoma model. J Invest Dermatol. 1998. 110:867–871.

26. Majumdar AS, Zolotorev A, Sammuel S, Tran K, Vertin B, Hall-Meier M, Antoni BA, Adeline E, Philip M, Philip R. Efficacy of herpes simplex virus thymidine kinase in combination with cytokine gene therapy in an experimental metastatic breast cancer model. Cancer Gene Ther. 2000. 7:1086–1099.

27. Toda M, Martuza RL, Rabkin SD. Combination suicide/cytokine gene therapy as adjuvants to a defective herpes simplex virus-based cancer vaccine. Gene Ther. 2001. 8:332–339.

29. Maraskovsky E, Chen WF, Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. J Immunol. 1989. 143:1210–1214.
30. Saito S, Bannerji R, Gansbacher B, Rosenthal FM, Romanenko P, Heston WD, Fair WR, Gilboa E. Immunotherapy of bladder cancer with cytokine gene-modified tumor vaccines. Cancer Res. 1994. 54:3516–3520.
31. Blaese RM, Ishii-Morita H, Mullen C, Ramsey J, Ram Z, Oldfield E, Culver K. In situ delivery of suicide genes for cancer treatment. Eur J Cancer. 1994. 30A:1190–1193.

32. Barba D, Hardin J, Sadelain M, Gage FH. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA. 1994. 91:4348–4352.

33. Gansbacher B, Bannerji R, Daniels B, Zier K, Cronin K, Gilboa E. Retroviral vector-mediated gamma-interferon gene transfer into tumor cells generates potent and long lasting anti-tumor immunity. Cancer Res. 1990. 50:7820–7825.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download