Abstract
We report a case of cat scratch disease caused by Bartonella henselae in Korea. A 25-yr-old woman developed left cervical lymphadenopathy with history of contact with a dog. The cervical lymphadenopathy persisted for 1 month and resolved gradually and spontaneously. Serologic test was not done during the acute stage of the disease. Immunofluorescent antibody test performed during the convalescent stage was positive for B. henselae. To confirm B. henselae infection, polymerase chain reaction (PCR) analysis using aspirates of cervical lymph node was performed and the presence of B. henselae DNA was demonstrated. This is the first reported case of cat scratch disease in Korea confirmed by PCR for B. henselae DNA .
Cat scratch disease (CSD) is a worldwide zoonosis caused by Bartonella henselae or possibly by Bartonella clarridgeiae (1-3). It is characterized usually a self-limiting regional lymphadenopathy, associated with a cat scratch or bite. Originally considered rare, it is now recognized as a common cause of lymphadenopathy in children and young adults (2). Classic systemic disease includes a cutaneous inoculation by a scratch or bite followed by a regional lymphadenopathy after a variable period, ranging from 1 to 8 weeks. The number of pet cats is increasing in developed countries including Korea. According to the increase in number of pet cats, zoonosis like CSD has risen as a health problem in human society. In the past most cases of CSD were diagnosed by clinical manifestations and intradermal reaction with specimens taken from patients before isolation of the causative organisms. Due to the difficulty of isolation of B. henselae from CSD patient, the diagnosis is usually based on serologic data and clinical history when informative. Recently polymerase chain reaction (PCR) is used as a confirmative method with biopsy or aspiration specimen of lymph nodes from CSD patients (4-6). In Korea, there is no reported case of CSD confirmed by PCR. This report deals with a case of CSD confirmed by PCR assay using different sets of primers.
A 25-yr-old previously healthy woman visited Sanggyepaik Hospital with high fever over 7 days and painful mass in the left neck. In spite of medication of oral antibiotics at a private clinic, her symptoms were not improved. She had been admitted to our hospital on 7 May 2004. She had been keeping a dog for 4 months before admission but had no history of contact with a cat.
On admission there were multiple palpable mass in the left neck area. The masses were 2 cm and 1.5 cm in diameter, and were tender. On physical examination liver and spleen was not palpable. There was no skin lesion or scratched wound in the face, extremity and trunk.
She had a white cell count of 3,490×109/L (neutrophil, 87%; lymphocyte, 8.6%; monocyte, 3.2%), with platelets 100×109/L, a hemoglobin of 12.1 g/dL. Blood chemistry revealed: AST 71 IU/L, ALT 62 IU/L, total bilirubin 0.3 mg/dL, BUN 8 mg/dL, creatinine 0.7 mg/dL. Laboratory findings showed elevated CRP, but ESR was 3 mm/hr. ANA and anti-dS DNA was negative. The computed tomography of the patient's neck showed multiple variable-sized lymph nodes (maximum 16×10 mm). The serum sample from the patient was tested for B. henselae antibodies by using a commercial immunofluorescent assay (Bartonella IFA IgG; Focus technologies, Cypress, CA, U.S.A.). The IgG titer was 1:64 positive. Aspiration cytology of lymph node of left neck revealed reactive hyperplasia.
The patient started receiving clindamycin intravenously for 6 days after lymph node aspiration. The fever and pain in the left neck area persisted during the treatment. Under the impression of reactive lymphadenitis she had been discharged without medication. During the outpatient clinic follow up her symptoms improved gradually without medication and completely recovered one month later. She had remained asymptomatic for 3 months.
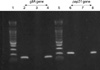
To prepare template DNA from the lymph node aspirate, QIAamp DNA Tissue Mini Kit (QIAGEN GmbH, Hilden, Germany) was used. B. henselae Huston-1 (ATCC 49882) DNA was used for positive control. We selected the primer sets (TN-1, TN-2, and IP) for the gltA gene used by Margolis et al. (5) and the primer sets (PAPn1, PAPn2, and PAPns2) for the pap 31 gene used by Zeaiter et al. (6). Seminested PCR protocols for amplification of the B. henselae gltA and pap31 genes were applied to the sample (Table 1). The size of the amplified DNA fragments was 139 bp and 211 bp for the gltA and pap31 genes respectively (6, 14). B. henselae DNA was detected from patient's lymph node aspirate (Fig. 1). PCR products were sequenced. For gltA gene, IP and TN-1 were used (5) and for pap31, PAPns2 and PAPns1 were used (6). They were sequenced at both directions with BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster, CA, U.S.A.). Sequencing products were resolved with ABI 3,730 XL autoanalyzer (Applied Biosystems, Foster, CA, U.S.A.). The sequences were aligned with the gltA or pap31 sequences available in GenBank for B. henselae isolates. The patient's PCR product for gltA had a consistent sequence of B. henselae and for pap31 gene showed a consistent sequence corresponding to main genogroup of B. henselae Houston (Fig. 2).
CSD, caused by Bartonella henselae, is a worldwide zoonosis associated with a variety of clinical manifestations. Typically, a nontender papule develops in the scratch line, three to 10 days after exposure, healing without scarring in two or three weeks. Regional lymphadenopathy follows in more than 80% of cases, but resolves usually within two to three months (7). Atypical CSD are reported up to 25% of cases (8). The manifestations of atypical CSD are fever of unknown origin, neuroretinitis, encephalopathy, hepatosplenic granuloma, juvenile rheumatoid arthritis. Nowadays, B. henselae infection is regarded as a common cause among patients with fever of unknown origin (9). Atypical presentations are considered as manifestations of Bartonella infection rather than CSD. The epidemiological and clinical characteristics of CSD have been well delineated in countries other than Korea (2, 8). Epidemiological and clinical study of CSD in Korea is rare (10). Domestic cats or dogs are the reservoirs for B. henselae and it is transmitted through scratches or bites (7, 11, 12). However, no history of animal contact can be elicited in small percentage of CSD patient.
Cat scratch disease is usually a self-limiting disease and does not require therapy. But, some patients with multi-system involvement may benefit from antibiotics treatment, so it is necessary to identify the organism rapidly by clinical laboratory assay. The diagnosis of CSD is made currently on the basis of clinical criteria in addition to a recent history of cat or dog exposure, a scratch or a flea bite plus bacteria culture, histologic examination of tissue biopsies and serologic test. Although serologic analysis by immunofluorescence or enzyme linked immnuosorbent assay is a useful tool for the diagnosis of B. henselae infection, the specificity of serological assay has been questioned due to the cross reactivity between B. henselae and other species (13). Also antigenic variability within the species could partly explain inconsistent results in the serological diagnosis of CSD.
PCR assays appear to be very useful in confirming clinically suspected CSD and have advantage of rapid diagnosis with the reliability since it is independent on the patient's humoral response. Recent studies relied on PCR amplification to improve diagnosis of CSD (4-6, 14). Earlier assays targeted amplification of 16S rRNA gene which is present in all bacteria with species polymorphism (14). PCR assay using the amplification of a portion of the citrate synthase gene (gltA) followed by TaqI restriction digest of the products is a sensitive tool for the detection of B. henselae DNA in tissue biopsy specimens and pus aspirates from lymph nodes of patients with CSD, but require large amounts of clinical material. The pap 31 gene encodes a major protein associated with a phage from B. henselae and allowed the classification of its strains into two clusters, B. henselae Houston-1 and B. henselae Marseille (6). Avidor et al. (14) demonstrated that PCR diagnosis of CSD from fine needle aspiration and primary lesion specimens can be minimally invasive and highly accurate, so precluding the necessity for excision biopsy. In this case, antibody titer to B. henselae was positive (1:64) and corresponding sequence to major genogroup B. henselae Houston was detected by PCR analysis of lymph node tissue by fine needle aspiration. PCR offers a rapid and specific means to detect the organism directly from clinical specimens in CSD patients. And it is more sensitive than isolation when performed on suitable clinical samples such as fresh or frozen lymph node tissues.
In conclusion, when presented with lymphadenopathy, physicians should inquire about recent cat, dog, or pets contact and/or animal scratches considering the possibility of CSD.
Figures and Tables
Fig. 1
Results of seminested polymerase chain reaction (PCR) for gltA gene and pap 31 gene of B. henselae. Lanes 2-4 for PCR of gltA gene (139 bp), lane 6-8 for PCR of pap31 gene (211 bp). Lane 1 and 5, DNA ladder marker (Bioneer, Daejeon, Korea); lane 2 and 6 positive control (Houston-1, ATCC 49882); lane 3 and 7, negative control; lane 4 and 8, lymph node tissue from the patient with cat scratch disease.
References
1. Anderson BE, Neuman MA. Bartonella spp. As emerging human pathogens. Clin Microbiol Rev. 1997. 10:203–219.

2. Carithers HA. Cat-scratch disease: an overview based on a study of 1,200 patients. Am J Dis Child. 1985. 139:1124–1133.
3. Regnery RL, Olson JG, Perkins BA, Bibb W. Serological response to "Rochalimaea henselae" antigen in suspected cat scratch disease. Lancet. 1992. 339:1443–1445.
4. Sander A, Posselt M, Bohm N, Ruess M, Altwegg M. Detection of Bartonella henselae DNA by two different PCR assays and determination of the genotypes of strains involved in histologically defined cat scratch disease. J Clin Microbiol. 1999. 37:993–997.

5. Margolis B, Kuzu I, Hermann M, Raible MD, Hsi E, Alkan S. Rapid polymerase chian reaction-based confirmation of cat scratch disease and Bartonella henselae infection. Arch Pathol Lab Med. 2003. 127:706–710.
6. Zeaiter Z, Fournier PE, Raoult D. Genomic variation of Bartonella henselae strains detected in lymph nodes of patients with cat scratch disease. J Clin Microbiol. 2002. 40:1023–1030.

7. Windsor JJ. Cat scratch disease: epidemiology, aetiology and treatment. Br J Biomed Sci. 2001. 58:101–110.
8. Murakami K, Tsukahara M, Tsuneoka H, Iino H, Ishida C, Tsujino K, Umeda A, Furuya T, Kawauchi S, Sasaki K. Cat scratch disease: analysis of 130 seropositive cases. J Infect Chemother. 2002. 8:349–352.

9. Jacobs RF, Schutze GE. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis. 1998. 26:80–84.

10. Chae MB, Lee JY, Kwak YG, Park SH, Lim HJ, Park SW, Chung MH, Kim MK, Kang JS. Prevalence of antibodies to Bartonella henselae and Bartonella quintana in Korean patients with lymphadenopathy. Korean J Infect Dis. 2002. 34:305–310.
11. Tsukahara M, Tsuneoka H, Iino H, Ohno K, Murano I. Bartonella henselae infection from a dog. Lancet. 1998. 352:1682.

12. Keret D, Giladi M, Kletter Y, Wientoub S. Cat scratch disease osteomyelitis from a dog scratch. J Bone Joint Surg Br. 1998. 80:766–767.
13. Sander A, Berner R, Ruess M. Serodiagnosis of cat scratch disease: response to Bartonella henselae in children and a review of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2001. 20:392–401.

14. Avidor B, Varon M, Marmor S, Lifschitz-Mercer B, Kletter Y, Ephros M, Giladi M. DNA amplification for the diagnosis of cat scratch disease in small quantity clinical specimens. Am J Clin Pathol. 2001. 115:900–909.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download