Abstract
The aim of this study was to assess the effectiveness of active intervention with antenatal maternal corticosteroid and antibiotics therapy in infants delivered between 24 and 28 weeks of gestation after premature rupture of membrane. This retrospective study included pregnant women complicated by preterm delivery at the Dong-A University Hospital from 1998 to 2002. Patients were divided into labor induction group 1 (n=20), observation group 2 (n=19), and medication group 3 (n=20). We evaluated the effects of prolongation of pregnancy and intervention with maternal corticosteroids and antibiotics therapy on perinatal and neonatal outcomes. Each group did not have a significant difference (p<0.05) in neonatal outcomes, such as respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, retinopathy of prematurity, pneumonia, bronchopulmonary dysplasia, and sepsis. The mean latency period was 4.7 days and 7.6 days in groups 2 and 3, respectively. Therefore, this study was unable to demonstrate any beneficial effects of corticosteroids in improving neonatal outcomes and prolongation of the latency period with antibiotics.
Preterm premature rupture of membranes (PPROM) is defined as rupture of the fetal membranes before the onset of labor at less than 37 weeks gestation. The incidence of PPROM is approximately 1% of all pregnancies and about 30% of all preterm deliveries (1, 2). Cox and associates (3) reported that this complication was identified in only a small number of pregnancies but its contribution to perinatal death was significant. Although a substantial amount of research has been performed to study the management of PPROM (4-8), no consensus on the management of this disease exists. Approximately 75% of these women will deliver within 1 week of presentation (4) and average time gained with treatment was about 10 days (6). The most common complications of neonates include respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), and sepsis. The maternal risks include the uterine infection and its sequela.
The primary entry point for neonatal morbidity and mortality among preterm infants is RDS and its complications, the most important risk factor (9). Liggins observed that lambs born preterm after exposure to corticosteroids in utero survived longer than lambs that did not (10). Liggins and Howie first described the antenatal administration of corticosteroids for the acceleration of fetal pulmonary maturity in 1972 (11). Subsequently, various randomized controlled trials confirmed the benefits of antenatal corticosteroids given to mothers who were at risk of premature delivery (9, 11-14), which, substantially decreased the risk of RDS in preterm newborns. Maternal administration of corticosteroids has served as the primary therapeutic approach to RDS prevention. On the basis of these numerous studies, the National Institutes of Health (NIH) concluded that antenatal corticosteroid therapy is indicated for women at risk of premature delivery with few exceptions (15). NIH also reports that antenatal corticosteroid therapy is effective, even on extremely low birth weights, however not conclusive because not enough studies have been completed. One study reported that the incidence of IVH, delivered between 24 and 28 weeks of gestation, was reduced in the betamethasone group (7). However, there was insufficient data on pregnancies of less than 28 weeks gestation to confidently state the limits of efficacy of antenatal corticosteroid therapy.
Several investigators observed that antibiotics reduced the incidence of intrauterine infection after PPROM, demonstrated the prolongation of gestation, and a decrease in the rate of neonatal complications (4, 16-25). Mercer et al. reported the benefits of prophylactic antibiotics on parameters such as a prolonged latency period, significant decreases in neonatal sepsis, pneumonia, and IVH (4). Egarter et al. (16) also confirmed a significant decrease in neonatal sepsis and IVH. The National Institute of Child Health and Human Development (NICHD) and the Maternal Fetal Medicine Network performed a double-blind randomized controlled trial to investigate if antibiotic therapy during expectant management for PPROM would reduce neonatal complications. The results confirmed a prolonged latency period and significant reductions of neonatal morbidity and mortality (4). However, the optimal and most effective treatment is still a difficult decision, especially for a second trimester ruptured membranes. Farooqi et al. (6) conducted a retrospective study on survival and a 2-yr outcome of second-trimester rupture of membranes with expectant management. The perinatal survival rate was 50% and 88% when membranes ruptured at 23 to 25 weeks and 26 to 28 weeks, respectively. Thus, the most important factor in neonatal prognosis may be the gestational age. Numerous studies demonstrated a steady increase in perinatal survival rate after second-trimester PPROM by the use of antenatal corticosteroids and also the benefits of antibiotic therapy (8, 26). However, the management with these drugs in second-trimester PPROM continues to be debated.
Therefore we evaluated the effectiveness of maternal corticosteroids and antibiotic therapy in infants delivered between 24 and 28 weeks of gestation complicated by PPROM, as well as any complications.
This retrospective study included 59 singleton pregnant women complicated by PPROM between 24 and 28 weeks of gestation at Dong-A University Hospital from January 1998 to December 2002. Estimated gestational age was determined by the last menstrual period, if a reliable date was present, and confirmed with ultrasonography. Those with an unclear history of the last menstrual period of those with a history of an irregular menstrual cycle, the dates were revised to match the ultrasonography checked before 20 weeks of gestational age. PPROM was diagnosed according to standard clinical criteria, including the patient's history, a presence of a vaginal pool, a positive nitrazine test, ferning, and the amounts of amniotic fluid estimated by ultrasonography. Cases involving multiple gestation, antepartum stillbirth, induced abortion, active labor defined as frequent uterine contractions with cervical change, suspected chorioamnionitis on admission, nonreassuring fetal heart rate patterns at admission, and birth of an infant with a major congenital malformation were excluded.
All patients were complicated by preterm delivery between 24 and 28 weeks after PPROM. We evaluated the effects of maternal dexamethasone therapy and antibiotics therapy on the perinatal and neonatal outcomes based on the data extracted from the medical records. After a patient was identified with PPROM and did not meet any of the exclusion criteria, we divided all patients into 3 groups, labor induction group (group 1, n=20), observation group (group 2, n=19), and medication group (group 3, n=20). Group 1 did not receive any other medication, but only induction of labor with oral prostaglandin. Group 2 patients only received close monitoring of fetal and maternal status while waiting for spontaneous labor. Group 3 received parenteral ampicillin therapy, 2.0 g intravenously every 8 hr for 7 days and dexamethasone therapy, 5 mg intramuscularly every 12 hr for a total of four doses. We used a single course of dexamethasone therapy in all patients. In group 2 and 3, we carried out fetal heart rate monitoring daily and performed ultrasound examination at least once a week for estimation of fetal size, amniotic fluid volume, and fetal biophysical profile. Serial checks of maternal white blood cell (WBC) count and C-reactive protein (CRP) level on admission were performed. Further speculum examinations were avoided except in the case of regular uterine contraction. All pregnancies were terminated in cases of suspected maternal infection such as increasing evidence of WBC count or CRP level, elevation of maternal body temperature above 37.2℃, excluding any other possible causes of fever, fetal tachycardia, other clinical evidence of chorioamnionitis, and abnormal fetal heart rate pattern. In group 1, continuous fetal heart rate monitoring was performed. Cesarean delivery was performed in case of fetal compromise in all 3 groups.
Based on the medical records, we evaluated the clinical characteristics of the mother and neonate such as maternal age, parity, cervical condition at admission, amniotic fluid index at admission, the latency from rupture-to-delivery interval, maternal body temperature before delivery, clinical symptoms of chorioamnionitis, gestational age, birth weight, 1 min and 5 min Apgar score, and WBC counts and CRP levels of neonates and those of mothers sampled at least 12 hr before delivery. Also, we evaluated the incidences of RDS, IVH, ROP, NEC, pneumonia, BPD and sepsis. RDS was diagnosed by a neonatologist and required intubation with oxygen therapy and the classic appearance on a chest radiograph. IVH was diagnosed by neurosonographic examination of the head and was graded according to Papile and colleagues (27) and ophthalmologic examinations were performed by ophthalmologist to check for ROP. Neonatal sepsis was diagnosed either by positive blood culture, or by a combination of clinical signs and laboratory findings, such as leucopenia, thrombocytopenia, and elevated CRP level. All deliveries were attended by a neonatologist and all infants were admitted to the neonatal intensive care unit.
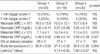
There are no significant differences among the groups with regards to maternal age, gestational age on admission, birth weight, amniotic fluid index on admission, cervical condition on admission, mode of delivery, and parity (p>0.05) (Table 1). Table 2 demonstrates that each group does not differ with respect to 1 and 5 min Apgar score, neonatal WBC count, or neonatal CRP level. However, we found significant differences in WBC count, CRP level and body temperatures of the mothers at delivery (p<0.05). Maternal WBC count and CRP level were much more different between groups 2 and 3 (p=0.007). We rationalize that antibiotics and corticosteroids can reduce the maternal morbidity from infections when delivery is delayed. Maternal body temperature at delivery of group 2 is higher than that of group 1 and 3 (p<0.05). The elevated body temperature may be related with maternal infection. The mean latency period of group 2 is 4.7 days and of group 3 is 7.6 days (p>0.05) (Table 2). The prolonged latency period of group 3 compared to group 2 may be attributed to the antibiotics; however, this was not statistically significant.
Table 3 reveals the neonatal complications of each group. In each of the complications, there are no statistically significant differences among the 3 groups (p>0.05). We cannot demonstrate that any other intervention, including antenatal corticosteroid and antibiotic therapy, even the prolongation of gestational age by waiting for spontaneous labor, improves the neonatal outcomes.
Women, who present with signs and symptoms of impending preterm delivery, are candidates for various interventions intended to improve infant outcomes. Interventions include antenatal corticosteroid therapy with or without antibiotic therapy. Both randomized studies and meta-analysis (28, 29) have shown that maternal corticosteroid administration reduces the risks of neonatal RDS, IVH, NEC and neonatal death, to a lesser extent, in preterm deliveries. However, the efficacy of impending preterm delivery is not well established because of insufficient data. Garite et al. (7), in a randomized controlled study, reported that antenatal corticosteroids did not improve perinatal outcomes and was not associated with maternal infection-related complications. They achieved statistically significant differences in the incidence of grade 3 and 4 IVH. Neonates born to women who received corticosteroids had a 3% incidence of IVH, whereas neonates born to women assigned to the placebo had a 25% incidence. However, the study groups were limited to babies delivered between 24 and 28 weeks gestation with intact membrane, not complicated by PPROM. Another study showed that antenatal corticosteroid therapy reduced the severity of IVH (30). In a one year prospective observational study, Chapman et al. (31) concluded that corticosteroid treatment did not have an apparent benefit. They focused on infants delivered at ≤29 weeks' gestation and weighing ≤1,000 g at birth whose mothers were seen for PPROM.
In our study, the incidences of RDS were 45%, 52.6%, and 45% in group 1, 2, and 3, respectively. Significant differences among the 3 groups were not detected.
Although IVH shows a higher incidence in group 3 (35%) than group 2 (26.3%), it was not statistically significant. The severity of IVH among the different groups was not analyzed.
The use of antenatal corticosteroid in patients with PPROM has been cautious, because of the fear that it may worsen an infection and its complications. One can hypothesize that antibiotic therapy would be important in decreasing the infectious morbidity associated with the immunocompromised state that results from steroid use in patients with PPROM. However, concomitant use of corticosteroids and antibiotics has been debated and is controversial. Leitich et al. (8) concluded that the beneficial effects of antibiotics in PPROM might be diminished when glucocoticoids are used concomitantly. Hence, a careful selection of patients who were likely to benefit from both therapies was needed (8). Lewis et al. (32) suggested that the use of broad-spectrum antibiotic before corticosteroids was beneficial to the babies without an increased risk of infectious morbidity. They hypothesized that if infection is the most likely etiology of PPROM, the antibiotics initially could be used to control the infection before administering a drug, corticosteroid, that could cause immumosuppression. They gave corticosteroid at least 12 hr after receiving antibiotics. However, the optimum period of duration of antibiotic therapy before administering steroids remains to be determined. In our study, we also administered antibiotics followed by corticosteroid, but we did not have a strict time of duration. Hence, this point remains to be studied further. However group 2 revealed an increased sign of infections such as elevation of WBC count, CRP level, and body temperature which may be due to the lack of antibiotic use. But, we could not estimate the effects of concomitant use of corticosteroid precisely. When comparing group 1 and group 3, there is no evidence of immunosuppression in group 3. The p-value of maternal WBC count, CRP level, and body temperature between group 1 and 3 are 0.382, 0.685, and 0.955, respectively, which are not statistically significant. Thus, the concomitant use of corticosteroid may not reduce the beneficial effects of antibiotics. The 2000 NIH consensus development conference recommended that repeat courses of antenatal corticosteroids should be reserved for patients enrolled in clinical trials. In our study, we use a single course method in all patients.
Numerous studies suggested that the use of antibiotics reduced the incidence of intrauterine infection after PPROM, other neonatal complications, and also could prolong the latency period (4, 16-25). Approximately 75% of women will be delivered within 1 week of presentation (4) and average time gained was about 10 days (6). In our study, the mean latency period of group 2 is 4.7 days and 7.6 days in group 3. A trend towards prolongation of the latency period when treated with antibiotics was noted, even though the difference is not statistically significant (p>0.05). Our average time gained is shorter than other previous reports (6). In one case, the maximum time gained was 30 days. This patient had a history of null parity and had adequate amniotic fluid volume.
Many centers have adopted some variation of the antibiotic regimen and its therapeutic duration. The NICHD used 2 days of parenteral antibiotics versus 5 days of oral antiboitics. Segel et al. (33) studied the effect of antibiotic treatment duration. They performed a randomized clinical trial comparing 3 days of ampicillin versus 7 days of ampicillin in patients with PPROM. They concluded that a shorter course of antibiotics could have the same effect on latency as a 7 day course without increasing of the incidence of infection-related complications and neonatal morbidity. We used ampicillin via the parenteral route as the primary antibiotic for a total duration of 7 days.
Unfortunately, we cannot make clear management recommendations for PPROM. Corticosteroids seem to be beneficial at gestational ages >28 weeks to decrease neonatal morbidity and mortality from RDS and IVH, but it seems to be ineffective between 24 and 28 weeks as shown in our study and other reports. If RDS is the primary entry point for neonatal morbidity and mortality among preterm infants, the important point of management is the prolongation of latency period. We can extend the time of latency by using antibiotics without increasing the evidence of infectious morbidity. However, we also used corticosteroid concomitantly with antibiotics, it is difficult from our data to estimate the effects of corticosteroids on our prolonged latency. A need exists for a prospective study to clarify this point.
Although it is very hard to make a clear conclusion in the management of second-trimester PPROM from our data, seems to increase the time of latency without increasing infectious morbidity. However, it is not effective on neonatal complications. This may be due a very small sample size. Thus, the further studies with large sample size are warranted.
Figures and Tables
Table 3
Neonatal complications of the 3 groups

RDS, Respiratory distress syndrome; IVH, Intraventricular hemorrhage; NEC, Necrotizing enterocolitis; ROP, Retinopathy of prematurity; BPD, Bronchopulmonary dysplasia; Group 1, labor induction group; Group 2, observation group; Group 3, medication group.
*, Fisher's exact test used to calculate p-values.
References
1. Parry A, Strauss JF III. Premature rupture of the fetal membranes. N Engl J Med. 1998. 338:663–670.

2. Arias F, Tomich P. Etiology and outcome of low birth weight and preterm infants. Obstet Gynecol. 1982. 60:277–281.
3. Cox SM, Williams ML, Leveno KJ. The natural history of preterm ruptured membranes: What to expect of expectant management. Obstet Gynecol. 1988. 71:558–562.
4. Mercer BM, Miodovnik M, Thurnau GR, Goldenberg RL, Das AF, Ramsey RD, Rabello YA, Meis PJ, Moawad AH, Iams JD, Van Dorsten JP, Paul RH, Bottoms SF, Merenstein G, Thom EA, Roberts JM, McNellis D. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes: randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997. 278:989–995.
5. Morales WJ, Talley T. Premature rupture of membranes at <25 weeks: A management dilemma. Am J Obstet Gynecol. 1993. 168:503–507.
6. Farooqi A, Holmgren PA, Engberg S, Serenius F. Survival and 2-year outcome with expectant management of second-trimester rupture of membranes. Obstet Gynecol. 1998. 92:895–901.

7. Garite TJ, Rumney PJ, Briggs GG, Harding JA, Nageotte MP, Towers CV, Freeman RK. A randomized, placebo-controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24 to 28 weeks' gestation. Am J Obstet Gynecol. 1992. 166:646–651.

8. Leitich H, Egarter C, Reisenberger K, Kaider A, Berghammer P. Concomitant use of glucocorticoids: a comparison of two metaanalysis on antibiotic treatment in preterm premature rupture of membranes. Am J Obstet Gynecol. 1998. 178:899–908.
9. Collaborative Group on Antenatal Steroid Therapy. Effects of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstet Gynecol. 1981. 141:276–287.
10. Liggins GC. Premature delivery of fetal lambs infused with glucocorticoids. J Endocrinol. 1969. 45:515–523.
11. Linggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome RDS in premature infants. Pediatrics. 1972. 50:515–525.
12. Papageorgiou AN, Desgranges MF, Masson M, Colle E, Shatz R, Gelfand MM. The antenatal use of betamethasone in the prevention of respiratory distress syndrome. A controlled double-blind study. Pediatrics. 1979. 63:73–79.
13. Taeusch HW, Frigoletto F, Kitzmiller J, Avery ME, Hehre A, Fromm B, Lawson E, Neff RK. Risk of respiratory distress syndrome after prenatal dexamethasone treatment. Pediatrics. 1979. 63:64–72.
14. Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990. 97:11–25.

15. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994. 12:1–24.
16. Egarter C, Leitich H, Karas H, Weiser F, Husslein P, Kaider A, Schemper M. Antibiotic treatment in preterm premature rupture of membranes and neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 1996. 174:589–597.

17. Ernest JM, Givner LB. A prospective, randomized placebo-controlled trial of penicillin in preterm premature rupture of membranes. Am J Obstet Gynecol. 1994. 170:516–521.

18. Whang JD, Roh CR, Yang SH, Lee JS, Kim WY, Yoo JD. Perinatal outcome with active expectant management of second-trimester rupture of membranes. Korean J Obstet Gynecol. 2001. 44:348–354.
19. Kenyon SL, Taylor DJ, Tarnow-Mordi W, Group OC. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001. 357:979–988.
20. Lockwood CJ, Costigan K, Ghidini A, Wein R, Chien D, Brown BL, Alvarez M, Cetrulo CL. Double-blind; placebo-controlled trial of piperacillin prophylaxis in preterm membrane rupture. Am J Obstet Gynecol. 1993. 169:970–976.

21. Lovett SM, Weiss JD, Diogo MJ, Williams PT, Garite TJ. A prospective, double-blind, randomized, controlled clinical trial of ampicillinsulbactam for preterm premature rupture of membranes in women receiving antenatal corticosteroid therapy. Am J Obstet Gynecol. 1997. 176:1030–1038.

22. Lee IS, Shin SM, Kang JA, Won HS, Lee PR, Kim A, Nam JH. The effect of antenatal conricosteroid on perinatal outcomes of preterm births. Korean J Obstet Gynecol. 2000. 43:863–870.
23. Mercer BM, Moretti ML, Prevost RR, Sibai BM. Erythromycin therapy in preterm premature rupture of the membranes: a prospective, randomized trial of 220 patients. Am J Obstet Gynecol. 1992. 166:794–802.

24. Mercer BM, Arheart KL. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995. 346:1271–1279.

25. Owen J, Groome LJ, Hauth JC. Randomized trial of prophylactic antibiotic therapy after preterm amnion rupture. Am J Obstet Gynecol. 1993. 169:976–981.

26. Goo BS, Chung JY, Kim JS, Kim SR, Lee SS, Won HS, Suh DS, Lee PR, Kim A. Benefits of antenatal corticosteroid in infants delivered before 33 weeks of gestation after premature rupture of membranes. Korean J Obstet Gynecol. 2004. 47:166–171.
27. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weight less than 1500gm. J Pediatr. 1978. 92:529–534.
28. Spinillo A, Capuzzo E, Ometto A, Stronati M, Baltaro F, Iasci A. Value of antenatal corticosteroid therapy in preterm birth. Early Hum Dev. 1995. 42:37–47.

29. Crowley PA. MRCOG. FRCPI. Antenatal corticosteroid therapy: A meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995. 173:322–335.

30. Silver RK, Vyskocil C, Solomon SL, Ragin A, Neerhof MG, Farrell EE. Randomized trial of antenatal dexamethasone in surfactant-treated infants delivered before 30 weeks' gestation. Obstet Gynecol. 1996. 87:683–691.

31. Chapman SJ, Hauth JC, Bottoms SF, Iams JD, Sibai B, Thom E, Moawad AH, Thurnau GR. Benefits of maternal corticosteroid therapy in infants weighing ≤1,000 grams at birth after preterm rupture of the amnion. Am J Obstet Gynecol. 1999. 180:677–682.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download