Abstract
This study evaluated the visual quality after wavefront-guided laser in situ keratomileusis (LASIK) for treating myopia. Thirty-two eyes with moderate myopia (-5.78~-2.17D) and 25 eyes with high myopia (-7.78~-6.17D) were prospectively reviewed. The contrast sensitivity (CS), glare and the total higher order aberrations (HOA) were measured before and 1 week, 1 month and 2 months after LASIK. The pupil diameter was measured at day- and night-time illumination. The CS and glare at all spatial frequencies were not reduced after wavefront-guided LASIK (p<0.05) and the difference between the moderate and high myopia group was not significant. No significant correlation was found between the amounts of myopia and the postoperative CS (p>0.05). The area under the log contrast sensitivity function (AULCSF) showed no correlation with the total HOA (r2=-0.071, p=0.612, between the daytime AULCSF and the total HOA with a 4 mm entrance pupil, r2=-0.176, p=0.260, between the nighttime AULCSF and the total HOA with a 6 mm entrance pupil). There was no decrease in CS and glare after wavefront-guided LASIK for myopia. In conclusion, wavefront-guided LASIK based on the individual ablation patterns is a good option for refractive surgery to improve the visual quality in both moderate and high myopia cases.
Laser in situ keratomileusis (LASIK) has a high rate of improving uncorrected Snellen visual acuity (1), however it can degrade the quality of vision, resulting in reports of reduced night vision clarity, glare, and halos (2). The contrast sensitivity test more effectively evaluates the visual quality over a range of sizes and daytime contrast levels (3) and is necessary to assess the visual performance in refractive surgery patients (4).
The increased higher order aberrations induced after LASIK is one reason for the reduced contrast sensitivity (5, 6). The contrast sensitivity after wavefront-guided LASIK using the ablation depth based on the individual higher order aberrations compared with standard LASIK after surgery showed a significantly improvement (7). The reduction in the contrast sensitivity was greater for higher amounts of myopia in standard LASIK (8).
However, previous studies for contrast sensitivity after wavefront-guided LASIK did not include high myopia patients. Most studies on the glare sensitivity after LASIK depended on questionnaires. The aim of this study was to evaluate the contrast sensitivity, glare sensitivity and total high order aberrations after wavefront-guided LASIK in both moderate and high myopia patients.
The patients were divided into 2 groups based on the preoperative spherical equivalent. Institutional review boards approved the study protocol, and all patients provided informed consent. The moderate myopia group included 32 eyes of 18 patients with -5.78~-2.17D. The high myopia group included 25 eyes of 14 patients with -7.78~-6.17D (Table 1). The uncorrected visual acuity, spherical equivalent, contrast sensitivity and glare sensitivity were measured preoperatively, and at 1 week, 1 month and 2 months postoperatively on patients undergoing wavefront-guided LASIK. The ocular total higher order aberration (HOA) was measured at 2 months postoperatively.
All LASIK procedures were performed by a single surgeon. Wavefront analysis and corneal ablation were performed using a Hartmann-Shack Aberrometer (Wavescan, VISX, Sunnyvale, CA, U.S.A.) and VISX Star S4 (VISX) excimer laser, respectively. In all patients, the individual aberrations gained including the higher order aberrations by Wavescan was programmed into the Star S4 laser to create a customized treatment. A flap thickness of 130 µm was created using a Moria M2 microkeratome (Moria, France). After surgery, ofloxacin 0.3% (Samil Pharm, Seoul, Korea) and fluorometholone 0.1% (Samil Pharm) were prescribed 4 times daily beginning one day after surgery for a one week.
The contrast sensitivity and glare were examined using a Visual Capacity Analyzer (VCA, L2 Informatique, France) with landolt rings as the optotypes in a darkened room with the monitor as the only light source. A standard 15-inch computer monitor was used, and the horizontal distance between the monitor and the eyes was 1 m. There were five spatial frequencies, each with 20 levels of 0.1-100% contrast: 3, 4.8, 7.5, 12, 19 (cycle/degree; cpd). The monitor illumination for the day and nighttime contrast sensitivity testing were 100 cd/m2 and 30 cd/m2, respectively. The measured levels were calculated as the log units, and the minimum contrast levels were recorded. The nighttime glare test used a VCA attaching light source (SB99, L2 Informatique, France) of 500,000 cd/m2 during 60 sec. If the patients did not read the landolt ring in the monitor as a result of a glare disturbance, the observer run the decrease button of VCA, which was automatically was recorded.
The pupil diameter was measured at the daytime (220 lux) and nighttime condition (5 lux) using a pupillometer (Colvard pupillometer, Oasis Medical, U.S.A.).
The Hartmann-Shack wavefront analyzer (Wavescan, VISX) was used to measure the ocular wavefront aberrations for a 4 mm and 6 mm entrance pupil. The total HOA was calculated from 3rd to the 6th order Zernike polynomials. The magnitudes of the coefficients of the Zernike polynomials are represented as the root mean square (RMS; in microns) and were used to show any ocular wavefront aberrations.
From the contrast sensitivity data obtained using this system, the area under the log contrast sensitivity function (AULCSF) was calculated according to the method reported by Applegate et al. (9). The log of the contrast sensitivity was plotted as a function of the log of the spatial frequency, and the third-order polynomials were fitted to the log spatial frequency limits of 0.48 (corresponding to 3 cpd) and 1.28 (19 cpd). The resulting value was defined as the AULSCF, which is a single quantity used to characterize the overall visual performance of the eye.
An independent t-test, analysis of variances (ANOVA), chi-square test and Pearson correlation analysis using SPSS software (SPSS Inc., Chicago, IL, U.S.A.) were used. A p-value <0.05 was considered significant.
Age, gender and preoperative pupil diameter in the daytime and nighttime did not differ between the two groups (p>0.05) (Table 1). Table 2 shows time course of the changes in the uncorrected visual acuity (UCVA). A UCVA of 20/20 or better was achieved by 96.0% in those with moderate myopia and by 94.1% in those with high myopia 2 months after surgery.
Fig. 1 shows the daytime contrast sensitivity at all spatial frequencies after wavefront-guided LASIK. The contrast sensitivity increased at 7.5 cpd at 1 month after wavefront-guided LASIK in the moderate myopia group (p=0.018). Fig. 2 shows the nighttime contrast sensitivity at all spatial frequencies after wavefront-guided LASIK. The contrast sensitivity increased at 7.5 cpd 2 month after wavefront-guided LASIK in the moderate myopia group (p=0.005). All other frequencies were not significantly different after wavefront-guided LASIK both in the daytime and nighttime (p>0.05).
The contrast sensitivity at all spatial frequencies did not differ in both groups (p>0.05, Fig. 3, 4). Table 3 shows correlation analysis between the amounts of myopia and postoperative 2 months contrast sensitivity at all spatial frequencies in the daytime and nighttime. No significant correlation was found between the ablation depth by wavefront-guided LASIK and the contrast sensitivity (p>0.05, Table 3).
In all patients with moderate myopia and high myopia, no correlation was found between the photopic pupil diameter and the daytime contrast sensitivity. The scotopic pupil diameter showed no correlation with the nighttime contrast sensitivity (p>0.05, Table 4).
The reduction in glare sensitivity was observed at 1 week and 1 month after surgery. However, this was recovered at 2 months postoperatively and the difference between the groups was not significantly different in the glare test (Table 5).
For all patients, the AULCSF in the daytime did correlate with the total HOA in the 4 mm entrance pupil (r2=-0.071, p=0.612). The correlation between the AULCSF in the nighttime and the total HOA in the 6 mm entrance pupil was not significant (r2=-0.176, p=0.260) (Fig. 5).
The visual acuity had improved after laser refractive surgery but most patients have reported blurring and glare symptoms (8). Their vision is also susceptible to the changes in illumination and contrast (2, 10). Therefore, a contrast sensitivity test is needed to more accurately and objectively evaluate the visual function (5, 11). This study was designed to evaluate the visual quality with a contrast sensitivity and glare test after wavefront-guided LASIK.
The visual acuity test determines the ability to resolve small details at a high contrast (12). It does not mean that an individual performs normally on all visual tasks, and the acuity is a poor predictor of the visual performance in certain daily perceptual tasks such as face recognition (12). On the other hand, contrast sensitivity discriminates the luminance differences between a material or an area in a space (13). As a sine-wave grating system, the contrast sensitivity is 3 or 5 times more sensitive than the letter acuity (13). This study used the VCA, which assesses overall visual function with difference modes (8). Lee et al. (8) reported that the reliability coefficient ranged from 89.1% to 99.8% under maximum background luminance. Under 3 cd/m2 background luminance, the reliability coefficient ranged from 97.5% to 100%.
Some studies have reported decreased low-contrast sensitivity after LASIK (10, 13-22). Chan et al. (4) related the contrast sensitivity reduction after LASIK to some optical factors (high order aberration) (5, 6). Eighty eight percent of the contrast sensitivity measurements improved 1 month after the wavefront-guided LASIK based on the individual high order aberration ablation pattern (7). However, previous studies did not include the high myopia patients. In standard LASIK, the reduction in the contrast sensitivity was greater for correction of higher amounts of myopia (1). In this study, contrast sensitivity did not differ between moderate and high myopia groups after surgery. In addition, the difference between the groups was not significant at all spatial frequencies. There was no significant correlation between the contrast sensitivity and level of myopia. Pop and Payette (23) reported that the AULCSF did not correlate with the total HOA (r=-0.11) after LASIK. Using a cutoff point of a total HOA of either 0.5, 0.6, or 0.7 µm, the independent t-test showed that the AULCSF did not differ between the lower and higher total HOA groups. These supports the hypothesis proposed in earlier studies in that a low wavefront aberration does not completely fit the entire visual performance (11, 24). In this study, the total HOA after wavefront-guided LASIK in most patients was <0.5 µm, and there was no correlation between the AULCSF and the total HOA.
A larger pupil causes a spurious resolution in an optically aberrant system and the pupil size is theoretically important in determining the optical quality of the retinal image and the visual performance (25, 26). However, previous studies reported that a low correlation between the contrast sensitivity and the glare symptoms after LASIK and the pupil size (27). This study also found no significant correlation between the contrast sensitivity and the pupil size in both the daytime and nighttime conditions. However, the number of cases in our study limited scotopic pupil was not larger than 7.0 mm. A large sample size with a wider range of pupil sizes will be necessary to confirm previous reports showing that the visual performance may demonstrate a decline in function related to the clearance zone compromised by a large pupil diameter (27).
El Danasoury (28) found, using a questionnaire, that 49% of eyes reported glare after LASIK with an optical zone of 5.5 mm. Several studies reported night-driving difficulties and glare ranging from 2% to 55.6% (29). The score of the nighttime glare symptoms with 75 lux after standard LASIK was 1.48±1.16 and 2.16±1.11 in those with moderate and high myopia, respectively (27). In all cases, the glare symptom was recovered 2 months after LASIK and there was no significant difference between the groups.
In conclusion, the reduction in the contrast sensitivity and glare was not caused after the wavefront-guided LASIK based on the individual higher order aberration in both moderate myopia and high myopia groups.
Figures and Tables
Fig. 1
The mean daytime (100 cd/m2) contrast sensitivity for the 5 spatial frequencies over time. Contrast sensitivity increased at 7.5 cpd at 1 month after wavefront-guided LASIK in moderate myopia (p=0.018). Contrast sensitivity of all other frequencies were not significantly different after wavefront-guided LASIK (p>0.05).

Fig. 2
The mean nighttime (30 cd/m2) contrast sensitivity for the 5 spatial frequencies over time. Contrast sensitivity increased at 7.5 cpd at 2 month after wavefront-guided in moderate myopia (p=0.005). Contrast sensitivity of all other frequencies were not significantly different after wavefront-guided LASIK (p>0.05).
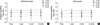
Fig. 3
Comparison of daytime (100 cd/m2) contrast sensitivity between moderate myopia and high myopia after wavefront-guided LASIK. Contrast sensitivity of all frequencies were not significantly different in the two groups (p>0.05).

Fig. 4
Comparison of nighttime (30 cd/m2) contrast sensitivity between moderate myopia and high myopia after wavefront-guided LASIK. Contrast sensitivity of all frequencies were not significantly different in the two groups (p>0.05).

Fig. 5
Correlation of area under the log contrast sensitivity Function (AULCSF) and total high order aberration (HOA) 2 month after wavefront-guided LASIK. (A) AULCSF in daytime (100 cd/m2) versus total HOA with 4 mm entrance pupil. (r2=-0.071, p=0.612), (B) AULCSF in nighttime (30 cd/m2) versus total HOA with 6 mm entrance pupil (r2=-0.176, p=0.260).
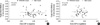
Table 3
Correlation between preoperative spherical equivalent and contrast sensitivity in postoperative 2 months

References
1. Sugar A, Rapuano CJ, Culbertson WW, Huang D, Varley GA, Agapitos PJ, de Luise VP, Koch DD. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002. 109:175–187.
2. Brunette I, Gresset J, Boivin JF, Pop M, Thompson P, Lafond GP, Makni H. Functional outcome and satisfaction after photorefractive keratectomy. Part 2: survey of 690 patients; the Canadian Refractive Surgery Research Group. Ophthalmology. 2000. 107:1783–1789.
3. Ginsburg AP, Evans DW, Cannon MW Jr, Owsley C, Mulvanny P. Large-sample norms for contrast sensitivity. Am J Optom Physiol Opt. 1984. 61:80–84.

4. Chan JW, Edwards MH, Woo GC, Woo VC. Contrast sensitivity after laser in situ keratomileusis; one-year follow-up. J Cataract Refract Surg. 2002. 28:1774–1779.
5. Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci. 2001. 42:1396–1403.
6. Marcos S, Barbero S, Llorente L, Merayo-Lloves J. Optical response to LASIK surgery for myopia from total and corneal aberration measurements. Invest Ophthalmol Vis Sci. 2001. 42:3349–3356.
7. Kaiserman I, Hazarbassanov R, Varssano D, Grinbaum A. Contrast sensitivity after wave front-guided LASIK. Ophthalmology. 2004. 111:454–457.

8. Lee HK, Koh IH, Choe CM, Kim CY, Hong YJ, Seong GJ. Reproducibility of morphoscopic contrast sensitivity testing with the visual capacity analyzer. J Cataract Refract Surg. 2003. 29:1776–1779.

9. Applegate R, Hilmantel G, Howland HC. Area under the log contrast sensitivity function (AULCSF) in radial keratotomy (RK): gains and losses. OSA Tech Digest Series Vis Sci & Its Appl. 1997. 1:223–226.
10. Verdon W, Bullimore M, Maloney RK. Visual performance after photorefractive keratectomy; a prospective study. Arch Ophthalmol. 1996. 114:1465–1472.
11. Montés-Micó R, Alió JL, Muñoz G. Contrast sensitivity and spatial-frequency spectrum after refractive surgery. J Cataract Refract Surg. 2003. 29:1650–1651.

12. Heitzmann J, Binder PS, Kassar BS, Nordan LT. The correction of high myopia using excimer laser. Arch Ophthalmol. 1993. 111:1627–1634.
13. Hamer RD, Mayer DL. Albert DM, Jakobiec FA, editors. The development of spatial vision. Principles and Practice of Ophthalmology: Basic Sciences. 1994. Philadelphia: WB Saunders;578–602.
14. Niesen UM, Businger U, Schipper I. Disability glare after excimer laser photorefractive keratectomy for myopia. J Refract Surg. 1996. 12:267–268.

15. Bullimore MA, Olson MD, Maloney RK. Visual performance after photorefractive keratectomy with a 6-mm ablation zone. Am J Ophthalmol. 1999. 128:1–7.

16. Schlote T, Kriegerowski M, Bende T, Derse M, Thiel HJ, Jean B. Mesopic vision in myopia corrected by photorefractive keratectomy, soft contact lenses, and spectacles. J Cataract Refract Surg. 1997. 23:718–725.

17. Niesen U, Businger U, Hartmann P, Senn P, Schipper I. Glare sensitivity and visual acuity after excimer laser photorefractive keratectomy for myopia. Br J Ophthalmol. 1997. 81:136–140.

18. Gauthier CA, Holden BA, Epstein D, Tengroth B, Fagerholm P, Hamberg-Nystrom H. Assessment of high and low contrast visual acuity after photorefractive keratectomy for myopia. Optom Vis Sci. 1998. 75:585–590.

19. Katlun T, Wiegand W. Change in twilight vision and glare sensitivity after PRK. Ophthalmologe. 1998. 95:420–426.
20. Knorz MC, Hugger P, Jendritzka B, Liermann A. Twilight visual acuity after correction of myopia with LASIK. Ophthalmologe. 1999. 96:711–716.
21. Vetrugno M, Quaranta GM, Maino A, Mossa F, Cardia L. Contrast sensitivity measured by 2 methods after photorefractive keratectomy. J Cataract Refract Surg. 2000. 26:847–852.

22. Nakamura K, Bissen-Miyajima H, Toda I, Hori Y, Tsubota K. Effect of laser in situ keratomileusis correction on contrast visual acuity. J Cataract Refract Surg. 2001. 27:357–361.

23. Pop M, Payette Y. Correlation of wavefront data and corneal asphericity with contrast sensitivity after laser in situ keratomileusis for myopia. J Refract Surg. 2004. 20:678–684.

24. Smolek MK, Klyce SD. Zernike polynomial fitting fails to represent all visually significant corneal aberrations. Invest Ophthalmol Vis Sci. 2003. 44:4676–4681.

26. Walsh G, Charman WN. The effect of pupil centration and diameter on ocular performance. Vision Res. 1988. 28:659–665.

27. Lee YC, Hu FR, Wang IJ. Quality of vision after laser in situ keratomileusis: Influence of dioptric correction and pupil size on visual function. J Cataract Refract Surg. 2003. 29:769–777.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download