Abstract
Allergic airway diseases are related to exposure to atmospheric pollutants, which have been suggested to be one factor in the increasing prevalence of asthma. Little is known about the effect of ozone and diesel exhaust particulates (DEP) on the development or aggravation of asthma. We have used a mouse asthma model to determine the effect of ozone and DEP on airway hyperresponsiveness and inflammation. Methacholine enhanced pause (Penh) was measured. Levels of IL-4 and IFN-γ were quantified in bronchoalveolar lavage fluids by enzyme immunoassays. The OVA-sensitized-challenged and ozone and DEP exposure group had higher Penh than the OVA-sensitized-challenged group and the OVA-sensitized-challenged and DEP exposure group, and the OVA-sensitized-challenged and ozone exposure group. Levels of IFN-γ were decreased in the OVA-sensitized-challenged and DEP exposure group and the OVA-sensitized-challenged and ozone and DEP exposure group compared to the OVA-sensitized-challenged and ozone exposure group. Levels of IL-4 were increased in the OVA-sensitized-challenged and ozone exposure group and the OVA-sensitized-challenged and DEP exposure group, and the OVA-sensitized-challenged and ozone and DEP exposure group compared to OVA-sensitized-challenged group. Co-exposure of ozone and DEP has additive effect on airway hyperresponsiveness by modulation of IL-4 and IFN-γ suggesting that DEP amplify Th2 immune response.
The rapid increase in motor vehicular traffic and its emissions have paralleled a worldwide increase in the incidence of allergic diseases such as asthma and rhinitis. A steady increment of diesel-engine-powered cars increased concentration of diesel exhaust particulates (DEP) in the atmosphere of urban areas. Because DEP contain carbon nuclei that absorbed a variety of toxic compound, including polyaromatic hydrocarbons, quinines, and aldehydes (1, 2), estimating the risk of DEP has recently become a major worldwide health issue. DEP remains buoyant in the atmosphere, with a certain fraction being carried into lungs (3). Many studies have suggested that DEP may cause lung cancer, pulmonary fibrosis, edematous change, chronic alveolitis, and bronchitis (4-6). However, few studies have investigated the relationship of DEP to bronchial asthma, which have shown increasing prevalence in urban areas of the world (7, 8).
Ozone is an important component of the photochemical oxidation product of air pollution emitted from automobile engines, and attentions have been drawn to its potential adverse effects on respiratory health because of its potential toxic effects related to its oxidant properties (9). Acute ozone exposure is known to decrease pulmonary function, increase airway hyperresponsiveness (AHR), and induce airway inflammation (10-14). Co-exposure of mice to ozone and carbon black particles for 4 hr decreased alveolar macrophage phagocytosis and increased lung neutrophilia in an enhanced manner compared to the exposure of each pollutant alone. The mechanisms for the enhanced biologic effect may be that the carbon black particles act as a carrier for ozone to the areas of distal lung not accessible to ozone in the gaseous phase or that ozone alters the physicochemistry of the particulate from a nontoxic to a toxic form (15). The effects of ozone were amplified by co-exposure with urban dust (16).
Little is known about the effect of co-exposure of DEP and ozone on AHR and airway inflammation in asthma model. Therefore the aim of present study was to evaluate the effects of ozone and DEP co-exposure on airway inflammation and AHR in BALB/c mice.
Female BALB/c mice 5-6 weeks of age were obtained from Daehan Laboratories (Daejeon, Korea). The mice were maintained on ovalbumin (OVA) free diets. The mice were individually housed in rack-mounted stainless steel cages with free access to food and water. An OVA-induced allergic airway disease model of asthma with some modification was used (17). Briefly, mice were sensitized by means of intra-peritoneal injection of on day 1, 14 day with 10 µg of Grade V OVA (Sigma Chemical, St Louis, MO, U.S.A.) and 1 mg of aluminum potassium sulfate (Sigma Chemical) in 500 µL of saline solution. Vehicle control mice were sensitized with a suspension of aluminum potassium sulfate (1 mg) in saline solution (500 µL) and challenged with nebulized saline solution daily from days 21 to 23 (Fig. 1). Aerosol challenge was carried out on groups of up 20 mice in a closed system chamber attached to an ultrasonic nebulizer (NE-UO7; Omron Corporation, Tokyo, Japan) with an output of 1 mL/min and 1- to 5-µm particle size.
The engine used for preparation of DEPs was 4JB1 type (Isuzu Automatic Co., Tokyo, Japan), light-duty (2,740 mL), four-cylinder diesel engine. The engine was connected to an EDGY dynamometer (Meiden-Sya, Tokyo, Japan), was operated using a standard diesel fuel with speeds of 1,500 rpm under the load of 10 torque (kg/m). The exhaust was introduced into a stainless-steel dilutuion tunnel (300 mm Φ× 8,400 mm) in a constant-volume sampler system equipped to the end of the dilution tunnel. The temperature at the sampling point was below 50℃. The diameter of the particles was measured by an Anderson Air Sampler of the low-pressure type (18), and the mean diameter was 0.4 µm. Most of the shapes analyzed by a scanning electron microscope were globular. The details on the DEPs we used were previously described (19, 20). Mice were placed in exposure chamber and exposed to DEP 2,000 µg/µL for 1 hr and 2 ppm ozone for 3 hr after 1% (wt/vol) OVA challenge on days 21 to 23.
The mice housed in whole-body exposure chambers were exposed to ozone concentrations of 2 ppm for 3 hr (n=6/group) after 1% (wt/vol) OVA challenge on days 21 to 23. Ozone was generated with Sander Model 50 ozonizers (Sander, Eltze, Germany). The concentration of ozone within the chambers was monitored throughout the exposure with ambient-air ozone motors (Model 49C; Thermo Environmental Instruments Inc., Franklin, MA, U.S.A.). The air-sampling probes were placed in the breathing zone of the mice. The mean chamber ozone concentration (±SEM) during the 3 hr exposure period was 1.98±0.08 ppm. Temperature and humidity were maintained at a constant level within the chamber.
Airway responsiveness was measured in unrestrained, conscious mice 1 day after the last challenge, as previously described (21). Mice were placed in a barometric plethysmographic chamber (All Medicus Co., Seoul, Korea), and baseline readings were taken and averaged for 3 min. Aerosolized methacholine in increasing concentrations (from 2.5 to 50 mg/mL) was nebulized through an inlet of the main chamber for 3 min. Readings were taken and averaged for 3 min after each nebulization and enhanced pause (Penh) was determined. Penh, calculated as (expiratory time/relaxation time-1)×(peak expiratory flow/peak inspiratory flow) according to the manufacturers' protocol, is a dimensionless value that represents a function of the proportion of maximal expiratory to maximal inspiratory box pressure signals and a function of the timing of expiration. Penh is used as a measure of airway responsiveness to methacholine. Results are expressed as the percentage increase of Penh following challenge with each concentration of methacholine, where the baseline Penh (after saline challenge) is expressed as 100%. Penh values averaged for 3 min after each nebulization were evaluated.
BAL was performed immediately after the last measurement of airway responsiveness. The mice were deeply anesthetized intraperitoneally with 50 mg/kg of pentobarbital sodium and were killed by exanguination from the abdominal aorta. The trachea was cannulated with a polyethylene tube through which the lungs were lavaged four times with 1.0 mL of physiologic saline (4.0 mL total). The BAL fluid was filtered through wet 4×4 gauze. Trypan blue exclusion for viability and total cell count was performed. The BAL fluid was centrifuged at 150×g for 10 min. The obtained pellet was immediately suspended in 4 mL of physiologic saline, and total cell numbers in the BAL fluid were counted in duplicate with hemocytometer (improved Neubauer counting chamber). Then, a 100 µL aliquot was centrifuged in a cytocentrifuge (Model 2 Cytospin; Shandon Scientific Co., Pittsburg, PA, U.S.A.). Differential cell counts were made from centrifuged preparations stained with Diff-quick, counting 500 or more cells in each animal at a magnification×1,000 (oil immersion).
The OVA-sensitized-challenged and ozone and DEP exposure group had significantly higher methacholine-induced Penh than the sham group and the OVA-sensitized-challenged group (p<0.01, Fig. 2).
Numbers of total cells, the proportion of eosinophils, and neutrophils in BAL fluids were increased significantly in the OVA-sensitized-challenged and ozone and DEP exposure group than in the OVA-sensitized-challenged group and OVA-sensitized-challenged and ozone exposure group (Sham vs. OVA vs. OVA+Ozone vs. OVA+DEP vs. OVA+Ozone+DEP; eosinophils, 1.2±0.07% vs. 2.9±0.09% vs. 7.2±1.15% vs. 7.5±2.3% vs. 8.9±1.12%; p<0.05, neutrophils; 1.3±0.01% vs. 2.3±0.17% vs. 8.3±1.26% vs. 4.5±2.5% vs. 10.1±1.18%; p<0.05).
Level of IL-4 was increased in the OVA-sensitized-challenged and ozone exposure group and the OVA-sensitized-challenged and DEP exposure group, and the OVA-sensitized-challenged and ozone and DEP exposure group compared to the OVA-sensitized-challenged group (p<0.05, Fig. 3A). Levels of IFN-γ were significantly decreased in the OVA-sensitized-challenged and DEP exposure group and the OVA-sensitized-challenged and ozone and DEP exposure group compared to the OVA-sensitized-challenged and ozone exposure group (p<0.05, Fig. 3B).
The important finding of this study was that the methacholine-induced Penh, and IL-4, neutrophils and eosinophils in BAL fluids were increased in the allergen-sensitized-challenged and ozone and DEP exposure group than in the allergen-sensitized-challenged and ozone exposure group. These findings suggest that ozone and DEP may have additive effect on AHR in murine asthma model and the mechanism responsible for increased AHR by DEP and ozone exposure might be a contribution of Th2 immune responses.
Air pollution is a complex mixture of substances. Particles might exacerbate asthma that act indirectly by amplifying the allergic airway inflammation and increase directly airway responsiveness to common allergen. Ozone is a pollutant that coexists with particulates to a varying extent. We hypothesized that a co-exposure of DEP and ozone would exacerbate the airway inflammation and AHR in asthma patients. Therefore we evaluated the effect of co-exposure air pollutants on murine asthma model. Intranasal administration of DEP increased AHR to acetylcholine in both A/J and C57Bl/6 mice (22). The organic compounds of diesel exhaust particles (DEP-PAHs) caused AHR (23). The authors reported that Penh, an index of airway obstruction, was increased in a dose-dependent manner in mice exposed to ozone and persists for at least 72 hr (24, 25). In this study ozone have increased AHR to methacholine in the ozone-exposed group compared to that of OVA group. Also ozone plus DEP have more increased AHR indicating that DEP and ozone have additive effect of AHR.
Levels of IL-4 were increased significantly in the OVA-sensitized-challenged ozone exposure group and the OVA-sensitized-challenged DEP exposure group and the OVA-sensitized-challenged and ozone and DEP exposure group compared to the OVA-sensitized-challenged group. These results are consistent with those of Fujimaki et al. (26, 27), who reported that the adjuvant effect of DEP on IL-4 production and allergen induced IgE in mice challenged with allergen by the inhalation route, and that DEP effects on cytokine production in mice challenged intratracheally with 100 µg DEP for 6 wks: IL-2, IL-4, IL-5, and GM-CSF levels were increased compared with control animals. IFN-γ were significantly decreased in the OVA-sensitized-challenged and DEP exposure group and the OVA-sensitized-challenged and ozone and DEP exposure group compared to the OVA-sensitized-challenged and ozone exposure group. These results are consistent with the results with Diaz-Sanchez et al. that ragweed plus DEP challenge had a pronounced inhibitory effect on IFN-γ (28, 29).
Macrophages, eosinophils, neutrophils, and lymphocytes were significantly increased in BAL fluid from the animals challenged with DEP plus antigen compared to the animals treated with either agent alone (27, 30-32). DEP aggravated OVA-induced airway inflammation characterized by infiltration of eosinophils and lymphocytes and an increase in goblet cells in bronchial epithelium. In this study, eosinophils and neutrophils were increased in the OVA-sensitized-challenged and ozone and DEP exposure group compared to OVA-sensitized-challenged group, suggesting that eosinophils and neutrophils may play an important role in the pathogenesis of AHR of murine asthma model. Also DEP and ozone may be additive effect of airway inflammation.
The mechanism responsible for increased AHR of the DEP exposed to ozone is being speculative. Ambient concentration of ozone can increase the biological potency of DEP. The ozonized DEP may play a role in the induction of lung responses by ambient particulate matter (33). In this murine model of asthma co-exposure of ozone and DEP have increased AHR compared with that of ozone exposure, and increase of IL-4 and decrease of IFN-γ compared with that of ozone exposure indicating that balance of Th1/Th2 cytokine has a role in AHR of DEP exposed asthma model.
In conclusion, DEP and ozone have additive effect on AHR in a murine model of asthma via Th1/Th2 modulating activity.
Figures and Tables
 | Fig. 1Schematic diagram of the experimental protocol. Mice were sensitized on days 1 and 14 by intraperitoneal injection of OVA emulsified in 1 mg aluminum hydroxide. On days 21, 22, and 23 after the initial sensitization, the mice were challenged for 30 min with an aerosol of 1% (wt/vol) OVA in saline (or with saline as a control) using an ultrasonic nebulizer. The mice housed in whole-body exposure chambers were exposed to ozone concentrations of 2 ppm for 3 hr (n=6/group) and DEP 2,000 µg/µL for 1 hr after 1% (wt/vol) OVA challenge on days 21 to 23. |
 | Fig. 2The effect of ozone and DEP on airway responsiveness in OVA-sensitized and challenged mice. Airway responsiveness was measured 24 hr after the last challenge in mice. Airway responsiveness to aerosolized methacholine was measured in unrestrained, conscious mice. Mice were placed in the main chamber of a barometric plethysmograph and nebulized first with saline and then with increasing doses (from 2.5 to 50 mg/mL) of methacholine for 3 min for each nebulization. Readings of breathing parameters were taken for 3 min after each nebulization, during which time Penh values were determined. Data represent mean±SEM from six independent experiments. *p<0.05 versus sham; †p<0.01 vs. sham group and the OVA-sensitized/OVA-challenged group, ‡p<0.01 vs. sham group and the OVA-sensitized/OVA-challenged group and the OVA-sensitized/ozone-exposure group, and the OVA-sensitized/DEP-exposure group. |
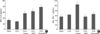 | Fig. 3The effect of ozone and DEP on IL-4, IFN-γ levels in BAL fluids of OVA-sensitized and challenged mice. Enzyme immunoassay of IL-4 (A), IFN-γ (B). Sampling was performed 72 hr after the last challenge in mice treated as described in Fig. 1. Bars represent mean±SEM from six independent experiments. *p<0.05 versus sham group. †p<0.05 versus the OVA-sensitized/ozone exposure group. |
References
1. Scruetzle D, Lewtas J. Bioassy-directed chemical analysis in environmental research. Anal Chem. 1986. 58:1060A–1075A.
2. Draper W. Quantification of nitro and dinitropolycyclic aromatic hydrocarbons in diesel exhaust particulate matter. Chromosphere. 1986. 15:437–447.
3. McClellan RO, Brooks AL, Cuddihy RG, Jones RK, Mauderly JL, Wolff RK. Lewtas IJ, editor. Inhalation toxicology of diesel exhaust particles. Toxicological effects of emissions from diesel engines. 1982. New York: Elsevier Scientific Publications;99–120.
4. Mauderly JL, Jones RK, Griffith WC, Henderson RF, McClellan RO. Diesel exhaust is a pulmonary carcinogen in rats exposed chronically by inhalation. Fundam Appl Toxicol. 1987. 9:208–221.

5. McClellan RO. Health effects of exposure to diesel exhaust particles. Annu Rev Pharmacol Toxicol. 1987. 27:279–300.

6. Ichinose T, Furuyama A, Sagai M. Biological effects of diesel exhaust particles (DEP) II. Acute toxicity of DEP introduced into lung by intratracheal instillation. Toxicology. 1995. 99:153–167.

7. Strachan DP, Anderson HR. Trends in hospital admission rates for asthma in children. BMJ. 1992. 304:819–820.

8. Haahtela T, Lindholm H, Bjorksten F, Koskenvuo K, Laitinen LA. Prevalence of asthma in Finnish young men. BMJ. 1990. 301:266–268.

9. Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Health effects of outdoor air pollution. Am J Respir Crit Care Med. 1996. 153:3–50.
10. Holtzman MJ, Fabbri LM, O'Byrne PM, Gold BD, Aizawa K, Walters EH, Alpert SE, Nadel JA. Importance of airway inflammation for hyperresponsiveness induced by ozone in dogs. Am Rev Respir Dis. 1983. 127:686–690.
11. Gordon T, Taylor BF, Amdur MO. Ozone inhibition of tissue cholinesterase in guinea pigs. Arch Environ Health. 1981. 36:284–288.

12. Basha MA, Gross KB, Gwizdala CJ, Haidar AH, Popovich J Jr. Bronchoalveolar lavage neutrophilia in asthmatic and healthy volunteers after controlled exposure to ozone and filtered purified air. Chest. 1994. 106:1757–1765.

13. Seltzer J, Bigby BG, Stulbarg M, Holtzman MJ, Nadel JA, Ureki IF, Leikauf GD, Goetzl EJ, Boushey HA. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol. 1986. 60:1321–1326.

14. Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, Kozumbo WJ, Becker S, House DE, McDonnell WF, Blomberg PA. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis. 1989. 139:407–415.

15. Jakab GJ, Hemenway DR. Concomitant exposure to carbon black particulates enhances ozone-induced lung inflammation and suppression of alveolar macrophage phagocytosis. J Toxicol Environ Health. 1994. 41:221–231.

16. Vincent R, Bjarnason SG, Adamson IY, Hedgecock C, Kumarathasan P, Guenette J, Potvin M, Goegan P, Bouthillier L. Acute pulmonary toxicity of urban particulate matter and ozone. Am J Pathol. 1997. 151:1563–1570.
17. Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994. 24:73–80.

18. Anderson AA. A sampler for respiratory health hazard assessment. Am Ind Hyg Assoc J. 1966. 27:160–165.
19. Sagai M, Furuyama A, Ichinose T. Biologic effects of diesel exhaust particles (DEP): III. Pathogenesis of asthma-like symptoms in mice. Free Radic Biol Med. 1996. 21:199–209.
20. Ohtoshi T, Takizawa H, Okazaki H, Kawasaki S, Takeuchi N, Ohta K, Ito K. Diesel exhaust particles stimulate human airway epithelial cells to produce cytokines relevant to airway inflammation in vitro. J Allergy Clin Immunol. 1998. 101:778–785.

21. Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997. 156:766–775.

22. Ohta K, Yamashita N, Tajima M, Miyasaka T, Nakano J, Nakajima M, Ishii A, Horiuchi T, Mano K, Miyamoto T. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: essential role of GM-CSF. J Allergy Clin Immunol. 1999. 104:1024–1030.

23. Fahy O, Hammad H, Senechal S, Pestel J, Tonnel AB, Wallaert B, Tsicopoulos A. Synergistic effect of diesel organic extracts and allergen Der p 1 on the release of chemokines by peripheral blood mononuclear cells from allergic subjects: involvement of the map kinase pathway. Am J Respir Cell Mol Biol. 2000. 23:247–254.
24. Jang AS, Choi IS, Kim SW, Song BC, Yeum CH, Jung JY. Airway obstruction after acute ozone exposure in BALB/c mice using barometric plethysmography. Korean J Intern Med. 2003. 18:1–5.

25. Jang AS, Choi IS, Koh YI, Park CS, Lee JS. The relationship between alveolar epithelial proliferation and airway obstruction after ozone exposure. Allergy. 2002. 57:737–740.

26. Fujimaki H, Nohara O, Ichinose T, Watanabe N, Saito S. IL-4 production in mediastinal lymph node cells in mice intratracheally instilled with diesel exhaust particulates and antigen. Toxicology. 1994. 92:261–268.

27. Fujimaki H, Saneyoshi K, Shiraishi F, Imai T, Endo T. Inhalation of diesel exhaust enhances antigen-specific IgE antibody production in mice. Toxicology. 1997. 116:227–233.

28. Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances in vivo nasal ragweed specific IgE and shews cytokine production to a T helper cell-type pattern. J Immunol. 1997. 158:2406–2413.
29. van Zijverden M, van der Pijl A, Bol M, van Pinxteren FA, de Haar C, Penninks AH, van Loveren H, Pieters R. Diesel exhaust, carbon black, and silica particles display distinct Th1/Th2 modulating activity. Toxicol Appl Pharmacol. 2000. 168:131–139.

30. Takano H, Yoshikawa T, Ichinose T, Miyabara Y, Imaoka K, Sagai M. Diesel exhaust particles enhance antigen induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med. 1997. 156:36–42.
31. Ichinose T, Takano H, Miyabara Y, Yanagisawa R, Sagai R. Murine strain differences in allergic airway inflammation and immunoglobulin production by a combination of antigen and diesel exhaust particles. Toxicology. 1997. 122:183–192.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download