1. Braunwald E, Kloner RA. The stunned myocardium: Prolonged, postischemic ventricular dysfunction. Circulation. 1982. 66:1146–1149.

2. Ito BR, Tate H, Kobayashi M, Schaper W. Reversibly injured, postischemic canine myocardium retains normal contractile reserve. Circ Res. 1987. 61:834–846.

3. Buffington CW, Rothfield KP. Effects of intracoronary calcium chloride on the postischemic heart in pigs. Ann Thorac Surg. 1995. 59:1448–1455.

4. Przyklenk K. Pharmacologic treatment of the stunned myocardium: the concepts and the challenges. Coron Artery Dis. 2001. 12:363–369.

5. Garden DL, Granger DN. Pathophysiology of ischemia-reperfusion injury. J Pathol. 2000. 190:255–266.
6. Dauber IM, Van Benthuysen KM, McMurtry IF. Functional coronary microvascular injury evident as increased permeability due to brief ischemia and reperfusion. Circ Res. 1990. 66:986–998.

7. Kim YD, Fomsgaard JS, Heim KF, Ramwell PW, Thomas G, Kagan E, Moore SP, Coughlin SS, Kuwahara M, Analouei A. Brief ischemia-reperfusion induces stunning of endothelium in canine coronary artery. Circulation. 1992. 85:1473–1482.

8. Ohgoshi Y, Goto Y, Futaki S, Taku H, Kawaguchi O, Suga H. Increased oxygen cost of contractility in stunned myocardium of dog. Circ Res. 1991. 69:975–988.

9. Hashimoto T, Buxton DB, Krivokapich J, Hansen HW, Phelps ME, Schelbert HR. Responses of blood flow, oxygen consumption, and contractile function to inotropic stimulation in stunned canine myocardium. Am Heart J. 1994. 127:1250–1262.

10. Thandroyen FT, Muntz KH, Buja LM, Willerson JT. Alterations in beta-adrenergic receptors, adenylate cyclase, and cyclic AMP concentrations during acute myocardial ischemia and reperfusion. Circulation. 1990. 82:Suppl. II30–II37.
11. Kusuoka H, Porterfield JK, Weisman HF, Weisfeldt ML, Marban E. Pathophysiology and pathogenesis of stunned myocardium: depressed Ca2+ activation of contraction as a consequence of reperfusion induced cellular calcium overload in ferret hearts. J Clin Invest. 1987. 79:950–961.
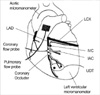
12. Vinten-Johannsen J, Johnston WE, Crystal GJ, Mills SA, Santamore WP, Cordell AT. Validation of local venous sampling within the at risk left anterior vascular bed in the canine left ventricle. Cardiovasc Res. 1987. 21:646–651.
13. Crystal GJ, Rock MH, Kim SJ, Salem MR. Effect of intracoronary infusions of amrinone and dobutamine on segment shortening, blood flow, and oxygen consumption in in situ canine hearts. Anesth Analg. 1994. 79:1066–1074.
14. Colucci WS, Denniss AR, Leatherman GF, Quigg RJ, Ludmer PL, Marsh JD, Gauthier DF. Intracoronary infusion of dobutamine to patients with and without severe congestive heart failure. Dose-response relationships, correlation with circulating catecholamines, and effect of phosphodiesterase inhibition. J Clin Invest. 1988. 81:1103–1110.

15. Edelson J, LeJemtel TH, Alousi AA, Biddlecome CE, Maskin CS, Sonnenblick EH. Relationship between amrinone plasma concentration and cardiac index. Clin Pharmacol Ther. 1981. 29:723–728.

16. Theroux P, Ross J Jr, Franklin D, Covell JW, Bloor CW, Sasayama S. Regional myocardial function and dimensions early and late after myocardial infarction in the unanesthetized dog. Circ Res. 1977. 40:158–165.

17. Carrozza JP Jr, Bentivegna LA, Williams CP, Kuntz RE, Grossman W, Morgan JP. Decreased myofilament responsiveness in myocardial stunning follows transient calcium overload during ischemia and reperfusion. Circ Res. 1992. 71:1334–1340.

18. Takayama M, Norris RM, Brown MA, Armiger LC, Rivers JT, White HD. Postsystolic shortening of acutely ischemic canine myocardium predicts early and late recovery of function after coronary artery reperfusion. Circulation. 1988. 78:994–1007.

19. Schlack W, Ebel D, Thamer V. Effect of inotropic stimulation on the synchrony of left ventricular wall motion in a dog model of myocardial stunning. Acta Anaesthesiol Scand. 1996. 40:621–630.

20. Dalmas S, Marsch SC, Philbin DM, Gavaghan DJ, Ryder WA, Foex P. Effects and interactions of myocardial ischemia and alterations in circulating blood volume on canine left ventricular diastolic function. Br J Anaesth. 1996. 76:419–427.
21. Chiu WC, Kedem J, Scholz PM, Weiss HR. Regional asynchrony of segmental contraction may explain the "oxygen consumption paradox" in stunned myocardium. Basic Res Cardiol. 1994. 89:149–162.
22. Parent R, Al-Obaidi M, Lavallee M. Nitric oxide formation contributes to beta-adrenergic dilation of resistance coronary vessels in conscious dogs. Circ Res. 1993. 73:241–251.

23. Mori K, Takeuchi S, Moritoki H, Tsuchiya K, Nakaya Y, Matsuoka S. Endothelium-dependent relaxation of rat thoracic aorta by amrinone-induced nitric oxide release. Eur Heart J. 1996. 17:308–316.

24. Stahl LD, Weiss HR, Becker LC. Myocardial oxygen consumption, oxygen supply/demand heterogeneity, and microvascular patency in regionally stunned myocardium. Circulation. 1988. 77:865–872.

25. Totaro RJ, Raper RF. Epinephrine-induced lactic acidosis following cardiopulmonary bypass. Crit Care Med. 1997. 25:1693–1699.

26. Warltier DC, Al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988. 69:552–565.

27. Gurevicius J, Holmes CB, Salem MR, Abdel-Halim A, Crystal GJ. The direct effects of enflurane on coronary blood flow, myocardial oxygen consumption, and myocardial segmental shortening in in situ canine hearts. Anesth Analg. 1996. 83:68–74.

28. Yoo KY, Lee JU, Kwak SH, Im WM, Jeong CY, Chung SS, Yoon MH, Jeong SW, Park JT. Effects of intracoronary calcium chloride on regional oxygen balance and mechanical function in normal and stunned myocardium in dogs. Br J Anaesth. 2002. 88:78–86.

29. Cain BS, Meldrum DR, Meng X, Shames BD, Banerjee A, Harken AH. Calcium preconditioning in human myocardium. Ann Thorac Surg. 1998. 65:1065–1070.

30. Belo SE, Mazer CD. Effects of halothane and isoflurane on postischemic stunned myocardium in the dog. Anesthesiology. 1990. 73:1243–1251.








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download