Abstract
The search for alternative epicardial energy sources in the treatment of nonvalvular atrial fibrillation (AF) is a relatively new aspect of the evolving spectrum of Maze operations. We tested the hypothesis that epicardial microwave ablation produces identical results to those of the standard cryosurgical Maze. Fourteen consecutive patients with chronic AF underwent on-pump epicardial Maze procedures after routine cardiac surgery. The results were compared with those of 14 control patients selected from our Maze database of 280 patients. There were no differences in age, sex, cardiothoracic ratio, duration of AF, pump time, intensive care unit or hospital stays. The aortic cross clamp time with epicardial microwave was, however, shortened from 110 to 65 minutes (p=0.011). The recurrence rate of AF after discharge showed no significant difference between the two groups (14% vs. 15%, p=0.841). Epicardial microwave ablation might be a valuable alternative to the conventional cryosurgical Maze procedure, especially for those patients without associated mitral valve disease.
Atrial fibrillation (AF) is a complex arrhythmia assumed to result from multiple reentry circuits, which in some instances might be several decades old. The "Cox" Maze procedure has become the standard AF surgery (1-5). Numerous modifications to the original Maze procedure have since been described, reporting high sinus conversion rates (6-19). The concepts regarding the mechanism of AF have further evolved with studies indicating that intermittent AF originates from ectopic focuses in the pulmonary veins. Catheter ablation techniques or surgery involving isolation of mainly the pulmonary veins have been founded on these concepts (17-20). Because fibrillation surgery is an additional procedure to the main cardiac procedure, cardiopulmonary bypass and cross clamp times might be prolonged. Therefore, it is important that the selected energy source not only be capable of producing complete transmural lesion but also achieve it in the shortest time possible. Recently, radiofrequency and microwave energies have been extensively studied to investigate their suitability as alternative energy sources to cryothermia (21-30). An energy source that can be applied epicardially on the beating heart has the potential to not only minimize endocardial trauma but also to significantly reduce the aortic cross clamping time that would otherwise be necessary with conventional techniques (8, 28-31). Microwave is one such energy source that fits this description and as such it is currently being used to perform epicardial ablative surgery, either in the on- or off-pump state on patients undergoing cardiac surgery. Specifically it has been found to be useful in patients with AF undergoing off-pump beating-heart coronary artery bypass grafting (OPCAB) or in patients with lone AF, which previously would have otherwise been considered an over-invasive treatment option. Although some reports, despite these advantages, might point to a possibly lower success rate with epicardial ablation when comparing it to conventional endocardial cryothermia (9, 24, 25, 29), it has been well demonstrated that complete linear transmural thermal lesions can be created with either microwave or radiofrequency ablation provided proper techniques are employed (30).
The purpose of the present study was to compare the results of AF surgery with epicardial microwave ablation combined with or without routine valvular surgery and the cryosurgical Maze operation.
Between July 1997 and June 2003, 280 patients received the Maze procedure using cyrothermia (Cryo group, Frigitronics Cardiac Cryosurgical System 200; Frigitronics, Inc, Coopersurgical, Shelton, CT, U.S.A.) with or without concomitant mitral procedures. Since July 2003, 14 patients underwent epicardial microwave maze (Epi-MW group) using the Flex 4 microwave antenna (Afx Inc, Fremont, CA, U.S.A.). The selected 28 hospital survivors who were the subjects of the present study were divided into 14 matched pairs. The patients were matched for age, sex, duration of AF, cardiothoracic ratio, preoperative left atrial dimension, incidence of fine AF, and concomitant cardiac conditions (Table 1, 2). To prevent selection bias, the patients were matched only by the preoperative variables, not taking into consideration the postoperative results.
The procedures, which were followed by transesophageal echocardiography, were performed by a single surgeon. Subsequent echocardiographic data were collected at 1 week, 3 and 6 months, and 1 yr after surgery.
Surgical ablation was performed under total cardiopulmonary bypass with aortobicaval cannulation followed by opening of the left atrial and vena caval pericardial reflection. Microwave energy was applied with FLEX 4 microwave ablation probes (Afx Inc, Fremont, CA, U.S.A.). The energy level used was 65 watts with the duration of ablation of 90 sec for epicardial ablation in the beating-heart condition and 45 sec for endocardial and epicardial ablation in the arrested-heart state. With epicardial ablation in patients undergoing non-open-heart cardiac surgery, the left and right pulmonary veins were separately isolated and connected to each other with a connecting lesion. The left atrial appendage was amputated or stapled off, after which another ablation line was created epicardially to connect to the pulmonary venous ablation lines. Pulmonary venous epicardial pacing was performed within the area of the isolated pulmonary veins to confirm electrical conduction block. A right atrial ablation line was formed by creating a thermal lesion extending from the superior vena cava to the inferior vena cava (crista terminalis). In those patients undergoing AF surgery concomitant to open heart surgery, cardiopulmonary bypass was performed after double venous cannulation. AF surgery was initiated with a long right atrial incision from the tip of the right atrial appendage to the fossa ovalis. The right atrial isthmus and the line from the atrial incision to the tricuspid valve were then ablated. The left atrial procedure was initiated with a standard left atriotomy incision along the Waterston groove, and extended toward the left inferior pulmonary vein, constituting the superior connecting lesion between the two pulmonary veins. The lines toward the mitral annulus (left atrial isthmus) and coronary sinus were then ablated either endocardially or epicardially. A temporary epicardial pacing wire was placed at the transitional junction of the superior vena cava and the right atrium or at the left atrial roof. The second pacing wire was placed at the right atrial appendage close to the interventricular septum. The pacing wires were used to evaluate the atrial p-wave activity for the presence of conduction block as well as for detecting the appearance or onset of the p-wave in the postoperative period. The atrial pacing wires were also used to induce atrial fibrillation by rapid atrial pacing.
Amiodarone was used to treat supraventricular premature beats as well as to treat either recurrent AF or flutter. A loading atrial intravenous dose of 300 mg was initially administered followed by a continuous infusion of 900 mg every 24 hr. Maintenance amiodarone of 200-400 mg was administered orally every 12 hr until discharge. Although a small routine dose of diuretics was sometimes necessary to maintain fluid balance, with preservation of the right atrial appendage in the latter part of the series, frank pulmonary edema was no longer detected. Warfarin was used routinely except in epicardial off-pump beating heart Maze procedures in which left atrial incision was not performed.
The focus of this study was to provide observational data on a new or modified method of performing the Maze procedure involving epicardial microwave ablation and to assess the performance of the new surgical device. Continuous variables were expressed as means±standard deviation. The means were compared using the independent t-test and ANOVA. Differences of frequency were compared using Fisher's exact test and chi-square test. A p value less than 0.05 was considered statistically significant. The statistical analysis was performed using the SPSS 10.0 software package (SPSS Inc., Chicago, IL, U.S.A.).
There were no differences in age, sex, cardiothoracic ratio, left atrial dimension, and duration of AF. Cardiopulmonary bypass time, intensive care unit and hospital stays also showed no differences. Aortic cross clamp time was significantly less with microwave ablation (p=0.011, ANOVA) (Table 1, 3). Concomitant surgical procedures included coronary bypass in 7 patients (4 Epi-MW, 3 Cryo), valvular surgery in 21 (11 Epi-MW, 10 Cryo) and congenital heart surgery in 2 patients (1 Epi-MW, 1 Cryo) (Table 2). There was no mortality, stroke, myocardial infarction, or re-exploration for bleeding. No permanent pacemaker implantation was necessary in any case.
Sinus conversion occurred in 25 of 28 patients in the first month (12 Epi-MW, 13 Cryo), and in 26 of 28 patients (13 Epi-MW, 13 Cryo) by 6 months postoperatively. The conduction block between the pulmonary veins and the non-isolated left atrium was verified by intraoperative pulmonary vein epicardial pacing (Table 4). Recurrent AF occurred in 2 patients (1 Epi-MW, 1 Cryo) and in 14 and 15% of the patients after discharge (p=0.841, chi-square test) (Table 5). The aortic clamp time was significantly shortened with Epi-MW maze procedure compared to the Cryo maze procedure (p=0.011).
The conventional Maze procedure, though effective, is associated with certain limitations such as poor left atrial functional recovery and loss of effective atrial contraction. Several modifications were developed to overcome these shortcomings, and some of them showed good results for atrial functional recovery (4, 10-12, 31, 32). The evolution and application of more advanced concepts such as posterior atrial wall reduction combined with cryoablation were thought to be contributory (31, 32). Meanwhile, the introduction of new devices using alternative energy sources added benefits, which included simplification and reduction of the operative time without compromising the surgical outcome. Currently, radiofrequency and microwave devices have shown the most promise among the energy sources that have been looked into as potentially suitable alternatives to cryoablation as they are easy to use, less time consuming and conducive to good results (21-30). However, some have pointed out that epicardial lesions created with either radiofrequency or cryoablation might be less penetrative than endocardial lesions due to the cooling effect of circulating blood and the epicardial fat tissue that can block energy transfer, necessitating a longer duration of ablation. Still, the time consumed is much less than it would take with the cut and sew technique (21, 28). Positive studies using microwave by a completely epicardial approach have shown success rates similar to those achieved with cryoablation (21, 22, 32). Although the endocardial approach is theoretically more effective, the disadvantages of this approach can include the necessitation of cross clamping and the inability to allow electrophysiological conduction block confirmation prior to aortic cross-clamp release and to CPB weaning. Compared to some of the other energy sources, the advantages of microwave energy include the ability to create complete transmural lesions, highly localized tissue destruction, immunity to the detrimental effects of fat tissue on energy transfer, ease of creating linear lesions facilitating isolation of the pulmonary veins, and the ability to achieve complete ablation epicardially (30). Of note, the energy release is unidirectional, preventing collateral damage. This is particular importance to epicardial beating-heart ablation as ablation could be carried out from the dorsal part of the heart without further protective measures. Review of our own series has also shown good results with AF ablation using the epicardial beating-heart approach. It was especially useful in situations where the ablation was incomplete, as further ablation was possible with a still-beating heart. The traditional Maze operation, however, would require the re-institution of cardiopulmonary bypass and aortic cross-clamping. In view of the increasing population of elderly patients with AF and the ensuing complications, it appears that a large group of patients that otherwise would not have had the benefit of receiving AF surgery can receive this surgery, because AF surgery with this technology might now be viewed by surgeons as being less invasive and much simplified. Likewise, the epicardial Maze procedure can not only benefit patients undergoing beating-heart coronary artery bypass grafting but also patients undergoing valve surgery. However, it is important to consider that the Maze procedure can prolong cardiopulmonary bypass time, though the procedure with the epicardial approach is simplified. Consequently, in finally weighing the benefit in valve surgery patients, the disadvantages of the additional obligatory prolongation in the CPB time and the aortic cross-clamp time should be considered.
In this study, no microscopic or macroscopic examinations were performed on the thermal lesions. We cannot speculate on the effects of the ablation duration on either the lesion size or depth and the effects of the tissue thickness. Accurate determination of the influence of these factors would require supplementary animal studies. A further limitation is the small size of the study population which may not be enough to obtain sufficient confidence.
In conclusion, the results of the current study showed epicardial microwave ablation with subsequent other cardiac procedure to be equally effective in inducing recovery of sinus rhythm in those who have undergone a conventional Maze procedure by open-heart surgery. Furthermore, it was possible to perform the ablation in a less traumatic manner and in a shorter time without adding to the ischemic time. In the long term, we expect that a significant proportion of patients undergoing heart surgery with AF that would otherwise not have received AF surgery will receive ablative AF surgery with this new technology.
Figures and Tables
Table 2
Operation
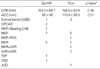
*, independent t-test.
ACC, aortic cross clamp time; Epi-MW, microwave epicardial ablation; CABG, coronary artery bypass graft; Cryo, cryoablation; OPCAB, off-pump coronary artery bypass; MVP, mitral valvuloplasty; PDA, patent ductus arteriosus; MVR, mitral valve replacement; AVR, Aortic valve replacement; TVP, tricuspid valvuloplasty; VSD, ventricular septal defect; ASD, atrial septal defect; Beating CAB, On pump beating heart CABG; AAR, ascending aorta replacement.
References
1. Cox JL, Schuessler RB, D'Agostino HJ Jr, Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991. 101:569–583.
2. Cox JL, Boineau JP, Schuessler RB, Jaquiss RD, Lappas DG. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J Thorac Cardiovasc Surg. 1995. 110:473–484.
3. Cox JL, Jaquiss RD, Schuessler RB, Boineau JP. Modification of the maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the maze III procedure. J Thorac Cardiovasc Surg. 1995. 110:485–495.
4. Imai K, Sueda T, Orihashi K, Watari M, Matsuura Y. Clinical analysis of results of a simple left atrial procedure for chronic atrial fibrillation. Ann Thorac Surg. 2001. 71:577–581.

5. Raanani E, Albage A, David TE, Yau TM, Armstrong S. The efficacy of the Cox/maze procedure combined with mitral valve surgery: a matched control study. Eur J Cardiothorac Surg. 2001. 19:438–442.

6. Melo J, Adragao P, Neves J, Ferreira M, Timoteo A, Santiago T, Ribeiras R, Canada M. Endocardial and epicardial radiofrequency ablation in the treatment of atrial fibrillation with a new intra-operative device. Eur J Cardiothorac Surg. 2000. 18:182–186.

7. Santiago T, Melo J, Gouveia RH, Neves J, Abecasis M, Adragao P, Martins AP. Epicardial radiofrequency applications: in vitro and in vivo studies on human atrial myocardium. Eur J Cardiothorac Surg. 2003. 24:481–486.

8. Benussi S, Pappone C, Nascimbene S, Oreto G, Caldarola A, Stefano PL, Casati V, Alfieri O. A simple way to treat chronic atrial fibrillation during mitral valve surgery: the epicardial radiofrequency approach. Eur J Cardiothorac Surg. 2000. 17:524–529.

9. Sie HT, Beukema WP, Ramdat Misier AR, Elvan A, Ennema JJ, Wellens HJ. The radiofrequency modified maze procedure. A less invasive surgical approach to atrial fibrillation during open-heart surgery. Eur J Cardiothorac Surg. 2001. 19:443–447.

10. Nitta T, Lee R, Schuessler RB, Boineau JP, Cox JL. Radial approach: a new concept in surgical treatment for atrial fibrillation I. Concept, anatomic and physiologic bases and development of a procedure. Ann Thorac Surg. 1999. 67:27–35.

11. Nitta T, Lee R, Watanabe H, Harris KM, Erikson JM, Schuessler RB, Boineau JP, Cox JL. Radial approach: a new concept in surgical treatment for atrial fibrillation II. Electrophysiologic effects and atrial contribution to ventricular filling. Ann Thorac Surg. 1999. 67:36–50.

12. Lee R, Nitta T, Schuessler RB, Johnson DC, Boineau JP, Cox JL. The closed heart MAZE: a nonbypass surgical technique. Ann Thorac Surg. 1999. 67:1696–1702.

13. Kosakai Y, Kawaguchi AT, Isobe F, Sasako Y, Nakano K, Eishi K, Tanaka N, Kito Y, Kawashima Y. Cox maze procedure for chronic atrial fibrillation associated with mitral valve disease. J Thorac Cardiovasc Surg. 1994. 108:1049–1055.

14. Kosakai Y, Kawaguchi AT, Isobe F, Sasako Y, Nakano K, Eishi K, Kito Y, Kawashima Y. Modified maze procedure for patients with atrial fibrillation undergoing simultaneous open heart surgery. Circulation. 1995. 92:Suppl 9. II359–II364.

15. Izumoto H, Kawazoe K, Kitahara H, Nasu M, Sasaki T, Kamata J, Tsuji I, Yagi Y. Can the maze procedure be combined safely with mitral valve repair? J Heart Valve Dis. 1997. 6:166–170.
16. Sueda T, Nagata H, Orihashi K, Morita S, Suehiro M, Hirai S, Matsuura Y. Efficacy of a simple left atrial procedure for chronic atrial fibrillation in mitral valve operation. Ann Thorac Surg. 1997. 63:1070–1075.
17. Sueda T, Imai K, Ishii O, Orihashi K, Watari M, Okada K. Efficacy of pulmonary vein isolation for the elimination of chronic atrial fibrillation in cardiac valvular surgery. Ann Thorac Surg. 2001. 71:1189–1193.

18. Takami Y, Yasuura K, Takagi Y, Ohara Y, Watanabe T, Usui A, Masumoto H, Sakai Y, Teranishi K. Partial maze procedure is effective treatment for chronic atrial fibrillation associated with valve disease. J Card Surg. 1999. 14:103–108.

19. Tuinenburg AE, Van Gelder IC, Tieleman RG, Grandjean JG, Huet RC, van der Maaten JM, Pieper EG, De Kam PJ, Ebels T, Crijns HJ. Mini-maze suffices as adjunct to mitral valve surgery in patients with preoperative atrial fibrillation. J Cardiovasc Electrophysiol. 2000. 11:960–967.

20. Szalay ZA, Skwara W, Pitschner HF, Faude I, Klovekorn WP, Bauer EP. Midterm results after the Mini-Maze procedure. Eur J Cardiothorac Surg. 1999. 16:306–311.

21. Spitzer SG, Richter P, Knaut M, Schuler S. Treatment of atrial fibrillation in open heart surgery-the potential role of microwave energy. Thorac Cardiovasc Surg. 1999. 47:Suppl 3. 374–378.
22. Knaut M, Spitzer SG, Karolyi L, Ebert HH, Richter P, Tugtekin SM, Schuler S. Intraoperative microwave ablation for curative treatment of atrial fibrillation in open heart surgery--the MICRO-STAF and MICRO-PASS pilot trial. MICROwave Application in Surgical treatment of Atrial Fibrillation. MICROwave Application for the Treatment of Atrial Fibrillation in Bypass-Surgery. Thorac Cardiovasc Surg. 1999. 47:Suppl 3. 379–384.
23. Williams MR, Stewart JR, Bolling SF, Freeman S, Anderson JT, Argenziano M, Smith CR, Oz MC. Surgical treatment of atrial fibrillation using radiofrequency energy. Ann Thorac Surg. 2001. 71:1939–1944.

24. Sie HT, Beukema WP, Misier AR, Elvan A, Ennema JJ, Haalebos MM, Wellens HJ. Radiofrequency modified maze in patients with atrial fibrillation undergoing concomitant cardiac surgery. J Thorac Cardiovasc Surg. 2001. 122:249–256.

25. Pasic M, Bergs P, Muller P, Hofmann M, Grauhan O, Kuppe H, Hetzer R. Intraoperative radiofrequency maze ablation for atrial fibrillation: the Berlin modification. Ann Thorac Surg. 2001. 72:1484–1491.

26. Manasse E, Colombo PG, Barbone A, Braidotti P, Bulfamante G, Roincalli M, Gallotti R. Clinical histopathology and ultrastructural analysis of myocardium following microwave energy ablation. Eur J Cardiothorac Surg. 2003. 23:573–577.

27. Mazzitelli D, Park CH, Park KY, Benetti FJ, Lange R. Epicardial ablation of atrial fibrillation on the beating heart without cardiopulmonary bypass. Ann Thorac Surg. 2002. 73:320–321.

28. Kubota H, Takamoto S, Morota T, Ohtsuka T, Motomura N, Kotsuka Y, Sudo K. Epicardial pulmonary vein isolation by cryoablation as concomitant cardiac operation to treat nonvalvular atrial fibrillation. Ann Thorac Surg. 2003. 75:590–593.

29. Benussi S, Nascimbene S, Agricola E, Calori G, Calvi S, Caldarola A, Oppizzi M, Casati V, Pappone C, Alfieri O. Surgical ablation of atrial fibrillation using the epicardial radiofrequency approach: mid-term results and risk analysis. Ann Thorac Surg. 2002. 74:1050–1057.

30. Maessen JG, Nijs JF, Smeets JL, Vainer J, Mochtar B. Beating-heart surgical treatment of atrial fibrillation with microwave ablation. Ann Thorac Surg. 2002. 74:S1307–S1311.

31. Lee JW, Choo SJ, Kim KI, Song JK, Kang DH, Song JM, Song H, Lee SK, Song MG. Atrial fibrillation surgery simplified with cryoablation to improve left atrial function. Ann Thorac Surg. 2001. 72:1479–1483.

32. Lee JW, Park NH, Choo SJ, Jo MS, Song H, Song MG. Surgical outcome of the maze procedure for atrial fibrillation in mitral valve disease: rheumatic vs degenerative. Ann Thorac Surg. 2003. 75:57–61.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download