Abstract
To determine the loading and maintenance dosage of glutathione (GSH) for patients suffering from reactive oxygen species (ROS) injury such as acute paraquat intoxication, a kinetic study of reduced GSH was performed in synchrony with that of cysteine (Cys), cystine (Cys2), and methionine (Met). Human subject's porticipitation was voluntary. The effective dose of Cys, Cys2, and Met against ROS in fibroblast cells generated by paraquat was assessed using laser scanning confocal microscopy. Both Cys and Met suppressed ROS in a dose-dependent manner at concentrations of 1-1,000 µM; the concentration required to suppress ROS by 50% was 10 µM for Cys and 50 µM for Met. Using metabolite kinetics with the assumption that Cys and Met are the metabolites of GSH, expected concentrations of Cys and Met of above 20 and 50 µM were estimated when GSH was administered at 50 mg/kg body weights every 205.4 min for Cys and 427.4 min for Met.
Accidental ingestion of paraquat is frequently fatal within a few days due to multiple-organ failure mediated by reactive oxygen species (ROS) (1). Over the past 30 yr, several methods for modifying the toxicity of paraquat have been examined: a) prevention of absorption by the gastrointestinal tract (2, 3), b) removal from the bloodstream (1, 4), c) prevention of accumulation in the lungs (5, 6), d) scavenging oxygen free radicals (7), and e) prevention of lung fibrosis (8, 9). Unfortunately, most of these methods have not proven effective, with the outcome already determined by the degree of exposure to paraquat.
Several sulfur-containing compounds have been examined as antioxidants in paraquat-induced lung injury due to their inherent antioxidant properties and an early observation that depletion of reduced glutathione (GSH) enhanced paraquat toxicity (10). Even though some studies have shown that alveolar type II cells can supplement endogenous synthesis of GSH with the uptake of exogenous GSH (11, 12), the antioxidant effectiveness of exogenously administered GSH is hindered by its instability when crossing cell membranes and its rapid hydrolysis in the circulation (13-15).
In circulation, GSH is degraded rapidly by gamma-glutamyltranspeptidase, an enzyme found on the extracellular surfaces of cells, yielding glutamate (Glu), cysteine (Cys), and glycine (Gly) (16). In some cells, degradation of GSH at the cell surface directly provides the cells with Cys required for GSH synthesis (17). Although Cys is a critical amino acid for the synthesis of GSH, it is sufficiently reactive in circulation for large amounts of Cys to be oxidized immediately to cystine (Cys2).
Recently we found that extracellular methionine (Met) is as strong an antioxidant as Cys against the intracellular ROS produced by paraquat. In man, this essential sulfur-containing amino acid is metabolized in the trans-sulfuration pathway (18). It is successively converted to S-adenosyl-methionine, S-adenosyl-homocysteine, and homocysteine. Homocysteine lies at a branch point from which sulfur metabolism can be controlled, either it can be remethylated to Met or converted to Cys via cystathione. The remethylation of homocysteine is catalyzed by betaine-homocysteine methyltransferase and 5-methyltetrahydro-folate-homocysteine methyltransferase. In this setting, Cys2 has been shown to increase the activity of betaine-homocysteine methyltransferase (19-21). In this regard, one of the potential functions of extracellular GSH is a source of both Cys and Met in the overall sulfur-containing amino acid balance. Aebi et al. (22) reported that Cys in plasma increased after the GSH infusion, but the plasma concentration of Cys plus Cys2 decreases.
Taking together the antioxidant effect of both Cys and Met, and the metabolic interrelationships between Met, Cys, and Cys2, we hypothesized that it would be more accurate to estimate the antioxidant effect of GSH based on the kinetics of each sulfur-containing amino acid. In order to prove this hypothesis, we observed the change of sulfur-containing amino acids concentration in blood, synchronized with the changes in GSH after the intravenous administration of GSH.
All of the reagents were obtained from Sigma (St. Louis, MO, U.S.A.) except where otherwise stated. This experimental study was approved by the Investigational Review Board at Soonchunhyang University Cheonan Hospital (Cheonan, Korea), and all human subjects provided written informed consent, and their participation was voluntary. Subjects ate a regular diet without alcohol and did not have any kinds of drugs including vitamins, for more than 3 days before the study.
Swiss 3T3 fibroblasts, obtained from American Type Culture Collection (ATCC CCL 92), were maintained at 37℃ in Dulbecco's modified Eagle's medium supplemented with 25 mM HEPES (pH 7.4), 10% (v/v) fetal bovine serum, 100 units/mL penicillin, and 100 mg/mL streptomycin. For experiments, cells were cultured on round coverslips in 12-well plates and then stabilized for 30 min with Dulbecco's modified Eagle's medium supplemented with 5 mg/mL apotransferrin, 1 mg/mL bovine serum albumin, 25 mM HEPES (pH 7.4), 2 mM glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin (serum-free medium).
The amount of intracellular ROS was measured as described in Koo et al. (23). Cultured cells on round coverslips were stabilized in serum-free medium without phenol red for at least 30 min, and then stimulated with paraquat for varying durations.
ROS generation in cells was assessed using the probe 2,7-dichlorofluorescein (DCF) (Molecular Probes, Eugene, OR, U.S.A.). Some of the cells were treated with various concentrations of antioxidants (GSH, Cys, Cys2, and Met) for 30 min prior to imaging. For the last 5 min of stimulation, the membrane-permeable diacetate form of the DCF: was added to the perfusate at a final concentration of 5 µM. Esterases within the cell cleave the acetate groups on DCF-diacetate, thus trapping the reduced probe intracellularly. Intensity values (confocal laser scanning microscope, LSM 510, Carl Zeiss, Germany) are reported relative to initial values after subtracting the background. In preliminary experiments, paraquat at 50-500 µM produced ROS in Swiss 3T3 fibroblasts in a dose-dependent manner at 30-60 min. The cells detached within 30 min when the paraquat concentration was over 600 µM, or when the incubation time was over 60 min at lower concentrations. Therefore, we selected the optimal condition for our experiments to be 40 min of incubation with 500 µM paraquat.
GSH (50 mg per kg of body weight, L-glutathione, reduced; Dong-A Pharmaceutical, Seoul, Korea) was infused into an antecubital vein over 10 min. Blood samples started from 6 o'clock in the morning after 12 hr overnight fasting through an indwelling intravenous catheter placed on other side of cubital vein, just before GSH administration (for basal level at time zero) and at 10, 20, 30, 60, 90, 120 and 240 min after GSH administration in seven male volunteers (medical students, aged 22 or 23 yr; body weight 65.5±4.5 kg). During the sampling, only water drinking was permitted. No side effect has observed after glutathione infusion.
Basal blood cell count, urinalysis and blood chemistry including BUN, creatinine, liver function test, and fasting blood sugar were normal in the all subjects. They ate a regular diet without alcohol and did not take any drugs including vitamins for at least 3 days before the study.
Samples were prepared and derivatized for HPLC analysis using procedures (with slight modifications) as described previously (24, 25). Briefly, 0.1 mL of serum was mixed with 0.1 mL of 25 mM dithiothreitol and 0.05 mL of 0.1 M Tris (pH 8.5) for the measurement of total GSH. The GSH-o-phthalaldehyde adducts were separated on a 4.6×250 mm Luna C18 column (5 µm, Phenomenex, Torrance, CA, U.S.A.) using two Waters 510 pumps, a 717 autosampler, and a 474 fluorescence detector (Milford, MA, U.S.A.), and fluorescence was detected at 420 nm with excitation at 340 nm. The amount of oxidized glutathione (GSSG) was obtained by subtracting the amount of GSH from the amount of total GSH.
Glu, Cys, Cys2, Met, and Gly were analyzed using the Pico-Tag method (Waters, Milford, MA, U.S.A.) after the serum was dried in a sample tube under vacuum (Korea Basic Science Institute, Daejeon, Korea) (26). Free-amino-acid samples were derived using derivatizing solution (ethanol:distilled H2O:triethylamine:phenylisothiocyanate at 7:1:1:1, v/v/v/v) for 15 min. The derivatized free amino acids were applied to a 30-cm Pico-Tag free-amino-acid-analysis column (3.9×300 mm) equilibrated with buffer A equipped with a Waters HPLC system (510 HPLC pump, 717 automatic sampler, 996 photodiode array detector, and Millennium 32 chromatography manager) and eluted with a linear gradient composed of buffer B (0%, 14%, 20%, 46%, and 100%) at a flow rate of 1 mL/min at 46℃. The absorbance at 254 nm was measured. Buffer A was 140 mM sodium acetate (6% acetonitrile), and buffer B was 60% acetonitrile.
Results are expressed as mean±SD unless stated otherwise. Intracellular ROS was measured in about 30 cells randomly selected from three separate experiments, and DCF fluorescence intensities of treated cells were compared with those of unstimulated control cells. Analysis of variance was used to detect differences in ROS between groups, and statistical significance was defined as a probability value of p<0.05.
The pharmacokinetics of GSH, GSSG, and total GSH were characterized by the peak plasma concentration (Cmax), elimination half time (t1/2), and the area under the plasma concentration-time curve (AUC) for the first 60 min (BA Calc 2002, KFDA, Ver 1.1.1). The elimination rate constant (ke) was determined by linear-regression analysis of the log-linear part of the concentration-time curve. The value of t1/2 was calculated as t1/2=ln (2/k). The AUC was calculated by the log-linear trapezoidal rule from 0 to 60 min after subtraction of the basal concentration. In order to avoid the influence of endogenous GSH, two concentrations (obtained at 120 and 240 min) were not included in calculating pharmacokinetic parameters because of the values less than basal level. The loading dose was calculated as the desired plasma level×volume of distribution at steady state, and the infusion rate as the desired plasma level×clearance (volume/unit time).
Our preliminary study found that Swiss 3T3 fibroblasts had a good reproducibility in both the production and the suppression of ROS, each done by paraquat and by antioxidants respectively. GSH at concentrations of 1-10 mM suppressed ROS in a dose-dependent manner, with 50% suppression was done by 5 mM GSH (Fig. 1A). Each of Cys, Cys2, and Met was observed to suppress ROS at various concentrations in dose-dependent manner: GSH at 1-10 mM, both Cys and Met at 1-1,000 µM, and Cys2 at 40-400 µM as shown in Fig. 1. 50% of the suppression was done by 5 mM GSH (Fig. 1A), by 10 µM Cys, 50 µM Met (Fig. 1B, C), and by 400 µM of Cys2 (data are not presented in figure).
The basal and peak plasma concentrations of GSH, GSSG, and total GSH following the infusion of GSH (50 mg per kg of body weight) are listed in Table 1. The elimination rate constants, the elimination half-life, the systemic clearance, and the apparent volumes of GSH, GSSG, and total GSH following the infusion of GSH are listed in Table 2. It is postulated that at the lowest effective plasma GSH concentration is 1 mM. Assuming this as the target concentration, the optimal dose of GSH can be determined from the pharmacokinetic parameters as follows. The loading dose (1.69 g/kg) was calculated by multiplying the volume of distribution administered by the target concentration. The optimal infusion rate was calculated by multiplying the target concentration by the clearance rate, and was 5.70 g/hr/kg. Because GSH has a very short half-life (of 10 min), it is difficult to maintain it at a therapeutic concentration.
Changes in the metabolites of GSH were presented in Table 1. Glu and Cys reached their peak concentrations at 10 min, and Met at 20 min. However, Cys2 increased gradually, peaked at 30 min, and then slowly decreased. The 10- and 20-min concentrations of Glu, Cys, and Met were significantly higher than their baseline values, but no significant change was observed in Cys2. The repeated ANOVA revealed statistically significant nonlinear relations between concentration and time for all four amino acids. The pharmacokinetic profile of Cys after subtracting the baseline concentration was summarized in Table 2. Using metabolite kinetics whilst assuming that Cys is a metabolite of GSH, and that AUC (Cys)single i.v. is 4108.4 µM·min after administering GSH at 50 mg per kg of body weight, the expected Cys concentration is over 20 µM when this dose is given every 205.4 min. In the same way, when it comes to Met, the concentration is over 50 µM when the same dose is given every 427.4 min.
The intracellular ROS produced by paraquat was suppressed by extracellular GSH at concentrations of 1-10 mM (Fig. 1A). This concentration range is three orders of magnitude higher than normal extracellular levels because the GSH concentration in circulation has known to be µM range. After intravenous administration, in agreement with a previous report (25), most of the GSH was oxidized to GSSG and disappeared immediately from circulation with a half-life of about 10 min. Pharmacokinetic investigations revealed that GSH at a loading dose of 1.69 g/kg and a maintenance dosage of 5.70 g/hr/kg are needed to reach 1 mM GSH which is a minimum requirement of extracellular concentration to suppress significantly the intracellular ROS. Considering that it is impossible to administer such a large amount of GSH in practical point of view, the intravenous administration of GSH would be an invalid treatment modality if GSH does not metabolized furthermore to other substances carrying antioxidant capacity.
In our study, five amino acids were measured over time: Glu, Cys, and Gly as metabolites of GSH, and Cys2 and Met as sulfur-containing compounds in plasma. Glu, Gly, and Cys reached peak concentrations at 10 min, Met peaked at 20 min, and Cys2 at 30 min. This finding suggests that GSH administered intravenously degraded immediately into the three amino acids. The intravenous infusion of GSH at 50 mg per kg of body weight increased the concentration of Cys to over 20 µM, which suppressed intracellular ROS by approximately 50% for about 60 min. Using metabolite kinetics with the assumption that Cys is a metabolite of GSH, Cys concentration over 20 µM would be achieved when GSH were administered at 50 mg/kg body weights every 205.4 min. In the same way, the Met concentration of 50 µM, which suppresses intracellular ROS by about 50%, would be achieved if GSH were administered at 50 mg per kg of body weights every 427.4 min.
Occasionally, the result of in vitro study used to be challenged when it is going to be extended to that of in vivo, because of the uncertainty of whether the results from in vitro experiment is also true in vivo. In that sense, our study raises fundamental questions to be answered. First of all, there may be difference in the intensity of ROS formation by paraquat and/or in ROS suppression by antioxidant, between in vitro cell line and cells in physiologic state. This is one of the subjects frequently argued in vitro study, which has to be overcome by a careful interpretation of other adjunct experiments. In this regard, our results that derived from the in vitro experiments and being extended to the in vivo experiments should be understood as relative ones rather than absolute ones, even the data are presented in numbers. The other problem is that in vitro and in vivo have the quite different metabolism system of GSH and amino acids.
The purpose of our current study was to find out how we could determine the appropriate dose of GSH for the patients with critical ROS injury such as acute paraquat intoxication. Our study just observed the epiphenomenon of sulfa-containing amino acid without intensive check-up on each amino acid metabolism respectively. During the initial 30 min after administration, the Cys2 level increased slowly and thereafter it decreased. The Met concentrations were higher than basal levels during the first 30 min and decreased as the Cys2 decreased (Fig. 1C). This implies that the increase in Cys2 stimulated Met synthesis. As we mentioned above the current study is not designed to determine the metabolic interrelationship among sulfur-containing amino acids. However, keeping in mind that methylation of homocysteine is an essential pathway to the formation of Met, it seems likely that GSH metabolites influence the enzymes involved in Met synthesis.
The remethylation of homocysteine is catalyzed by betaine-homocysteine methyltransferase and 5-methyltetrahydrofolate-homocysteine methyltransferase. In this setting, Cys2 is known to increase the activity of betaine-homocysteine methyltransferase-this is the so-called Cys2-sparing effect of Met, which is readily observed when Cys2 is added to a low-Met diet in animal models (20). Therefore, it seem reasonable that practical guidelines for the dosage of GSH should be derived from pharmacokinetic studies of sulfur-containing amino acids synchronized with the changes in GSH after the intravenous administration of GSH.
In conclusion, not being conclusive, our results provide us relevant clinical information. GSH is a useful antioxidant for the patients suffering from acute, critical injury mediated by ROS, and the recommended dose appears to be determined more reasonably when the metabolite of GSH is encountered.
Figures and Tables
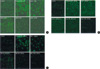 | Fig. 1Effect of glutathione, cysteine and methinine on the production of ROS by paraquat in Swiss 3T3 fibroblasts. (A) 10 mM of GSH suppressed ROS in dose dependant pattern. Complete suppression of ROS was observed at 5 mM of GSH. (B) Cys at 1-1,000 µM suppressed the production of ROS in a dose-dependent manner. Software quantification of the signal intensities produced the following values: 2.50±0.31 in paraquat group, 2.25±0.22 in 1 µM Cys, 1.30±0.17 in 10 µM Cys, and 1.20±0.15 in 100 µM Cys. Complete suppression was observed at 1,000 µM Cys. (a) and (b) denote statistically significant differences in comparison with control (a) and (b) paraquat groups, respectively. (C) Met at 1-1,000 µM suppressed the production of ROS in a dose-dependent manner. Software quantification of the signal intensities produced the following values: 2.50±0.31 in paraquat group, 2.40±0.30 in 1 µM Met, 2.05±0.25 in 10 µM Met, 1.19±0.15 in 100 µM Met, and 1.05±0.12 in 1,000 µM Met. (a) and (b) denote statistically significant differences in comparison with control (a) and paraquat (b) groups, respectively. |
References
1. Proudfoot AT, Prescott LF, Jarvie DR. Haemodialysis for paraquat poisoning. Hum Toxicol. 1987. 6:69–74.

2. Okonek S, Hofmann A, Henningsen B. Efficacy of gut lavage, hemodialysis, and hemoperfusion in the therapy of paraquat or diquat intoxication. Arch Toxicol. 1976. 36:43–51.

3. Meredith TJ, Vale JA. Treatment of paraquat poisoning in man: methods to prevent absorption. Hum Toxicol. 1987. 6:49–55.

4. Okonek S. Hemoperfusion in toxicology. Basic considerations of its effectiveness. Clin Toxicol. 1981. 18:1185–1198.

5. Lock EA, Smith LL, Rose MS. Inhibition of paraquat accumulation in rat lung slices by a component of rat plasma and a variety of drugs and endogenous amines. Biochem Pharmacol. 1976. 25:1769–1772.

6. Ross JH, Krieger RI. Structure-activity correlations of amines inhibiting active uptake of paraquat (methyl viologen) into rat lung slices. Toxicol Appl Pharmacol. 1981. 59:238–249.

7. Kitazawa Y, Matsubara M, Takeyama N, Tanaka T. The role of xanthine oxidase in paraquat intoxication. Arch Biochem Biophys. 1991. 288:220–224.

8. Webb DB, Williams MV, Davies BH, James KW. Resolution after radiotherapy of severe pulmonary damage due to paraquat poisoning. Br Med J (Clin Res Ed). 1984. 288:1259–1260.

9. Lin JL, Leu ML, Liu YC, Chen GH. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am J Respir Crit Care Med. 1999. 159:357–360.

10. Bus JS, Cagen SZ, Olgaard M, Gibson JE. A mechanism of paraquat toxicity in mice and rats. Toxicol Appl Pharmacol. 1976. 35:501–513.

11. Hagen TM, Brown LA, Jones DP. Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells. Biochem Pharmacol. 1986. 35:4537–4542.

12. Brown LA, Bai C, Jones DP. Glutathione protection in alveolar type II cells from fetal and neonatal rabbits. Am J Physiol. 1992. 262:L305–L312.

14. Jurima-Romet M, Barber RF, Demeester J, Shek PN. Distribution studies of liposome-encapsulated glutathione administered to the lung. Int J Pharm. 1990. 63:227–235.
15. Smith LJ, Anderson J, Shamsuddin M. Glutathione localization and distribution after intratracheal instillation. Implications for treatment. Am Rev Respir Dis. 1992. 145:153–159.

16. Reed DJ, Ellis WW, Meck RA. The inhibition of gamma-glutamyl transpeptidase and glutathione mtabolism of isolated rat kidney cells by L-(alpha S, 5S)-alpha-amino-3-chloro-4, 5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Biochem Biophys Res Commun. 1980. 94:1273–1277.
17. Rajpert-De Meyts E, Shi M, Chang M, Robison TW, Groffen J, Heisterkamp N, Forman HJ. Transfection with gamma-glutamyl transpeptidase enhances recovery from glutathione depletion using extracellular glutathione. Toxicol Appl Pharmacol. 1992. 114:56–62.
18. Mudd SH, Levy JL. Stanbury J.B, editor. Disorders of transsulphuration. Metabolic bases of inherited disease. 1982. 5th ed. New York: McGraw-Hill;522–559.
19. Stipanuk MH, Benevenga NJ. Effect of cystine on the metabolism of methionine in rats. J Nutr. 1977. 107:1455–1467.

20. Storch KJ, Wagner DA, Burke JF, Young VR. [1-13C; methyl-2H3] methionine kinetics in humans: methionine conservation and cystine sparing. Am J Physiol. 1990. 258:E790–E798.
21. Young VR, Wagner DA, Burini R, Storch KJ. Methionine kinetics and balance at the 1985 FAO/WHO/UNU intake requirement in adult men studied with L-[2H3-methyl-1-13C]methionine as a tracer. Am J Clin Nutr. 1991. 54:377–385.

22. Aebi S, Assereto R, Lauterburg BH. High-dose intravenous glutathione in man. Pharmacokinetics and effects on cyst(e)ine in plasma and urine. Eur J Clin Invest. 1991. 21:103–110.

23. Koo HY, Shin I, Lee ZW, Lee SH, Kim SH, Lee CH, Kang HS, Ha KS. Roles of RhoA and phospholipase A2 in the elevation of intracellular H2O2 by transforming growth factor-β in Swiss 3T3 fibroblasts. Cell Signal. 1999. 11:677–683.
24. Neuschwander-Tetri BA, Roll FJ. Glutathione measurement by high-performance liquid chromatography separation and fluorometric detection of the glutathione-orthophthalaldehyde adduct. Anal Biochem. 1989. 179:236–241.

25. Cereser C, Guichard J, Drai J, Bannier E, Garcia I, Boget S, Parvaz P, Revol A. Quantitation of reduced and total glutathione at the femtomole level by high-performance liquid chromatography with fluorescence detection: application to red blood cells and cultured fibroblasts. J Chromatogr B Biomed Sci Appl. 2001. 752:123–132.

26. Tarr GE. Shively J.E, editor. Methods of protein measurement. Microcharacterization. 1986. USA: Humana Press;155–194.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download