Abstract
Acute spinal cord injury (SCI) is two-step process that first involves the primary mechanical injury and then the secondary injury is induced by various biochemical reactions. Apoptosis is one of secondary SCI mechanisms and it is thought to play an important role for the delayed neuronal injury. The enhanced formation of nitric oxide (NO) via inducible nitric oxide synthase (iNOS) has been implicated in the pathogenesis of apoptosis in SCI. The level of .iNOS mRNA peaked at 6 hr after SCI and it declined until 72 hr after SCI in a rat model. Double-immunofluorescence staining revealed that iNOS positive cells were stained for ED-1, synaptophysin, GFAP, and oligodendrocyte marker. The terminal deoxynucleotidyl-transferase-mediated dUDP-biotin nick end-labeling (TUNEL) positive cell count was higher for the 72 hr post-SCI group than for the 24 hr post-SCI group. This cell count was also higher going in the caudal direction than in the rostral direction from the epicenter, and especially for the 72 hr group. Treatment with a selective iNOS inhibitor resulted in the reduction of TUNEL-positive cells at the lesion site. These findings suggest that nitric oxide generated by the iNOS of macrophages, neurons, oligodentrocytes, and astrocytes plays an important role for the acute secondary SCI that results from apoptotic cell death.
It is generally accepted that acute spinal cord ingury (SCI) is a two-step process involving primary and secondary injury mechanisms (1-3). The primary injury is caused by the initial mechanical insult due to the local deformation and energy transformation. The secondary cord injury is mediated by a cascade of biochemical and cellular processes that are initiated by the primary process and these secondary processes exacerbate tissue damage and limit the restorative processes. Various theories have been proposed concerning the biochemical and cellular mechanisms of secondary SCI. In the 1970s, the free radical mechanism was thought to be important in secondary SCI (4). In the 1980s, however, the roles of calcium, opiate receptors and lipid peroxidation became the focus of research. Modern research suggests that apoptosis, intracellular protein synthesis inhibition, and glutaminergic mechanisms play important roles in the mediation of secondary SCI (5). Apoptosis of neurons is caused by growth factor deprivation, ischemia, hyperthermia, hypercalcemia, excitotoxin, and active free radicals (6, 7). Understanding the apoptotic pathway, which is one of the mechanisms involved in delayed SCI, may contribute to the development of effective treatment strategies against the delayed neurologic deficit and the progression of cord injury.
Apoptosis in SCI was first recognized in 1997 as occurring in rats (8) and in humans (9). Emery et al. (9) reported that apoptosis was observed in the area adjacent to the injured site along the ascending and descending nerve bundles of the white matter, and they suggested that the oligodendrocytes are the major cell type to undergo apoptosis during spinal cord compression injury. Their proposal supported the report showing that oligodendroglial death may be the result of microglial activation (10).
Nitric oxide (NO) is known to be closely involved in the development of post-traumatic spinal cord cavitation as well as playing an important role in the development of the pathological process in vivo (11). NO-mediated cell injury occurs via both the necrotic and apoptotic pathways, according to the severity of the cellular damage (12). Hamada et al. have reported that the NO produced by inducible nitric oxide synthase (iNOS) is neurotoxic, whereas the NO produced by the nNOS is neuroprotective (13). A consensus has been reached that eNOS acts as a neuroprotective agent in the central nervous system (CNS) injury (14). However, there is still debates about the actions of iNOS and nNOS in CNS injury. The concentration range, the redox state, the cell type source, and the environment in which the NO is produced seem to determine the role of NO in the CNS (15). The aim of this study is to elucidate the actions of iNOS in parallel with the actions of nNOS, in the pathogenesis of secondary lesions after SCI.
Sprague-Dawley rats weighing 220-250 g were used as experimental animals. A modified version of Allen's method (16) was applied to create a contusion injury model. Rats were anesthetized with pentobarbital (50 mg/kg i.p.), immobilized in a stereotaxic instrument (Stoelting, Wood Dale, Illinois, U.S.A.) and laminectomy was performed at the T8-T10 level. Rats in control group received a laminectomy only (sham operation). In experimental group, a 10 g stainless steel cylinder with a flat tip of 2 mm diameter was dropped from a height of 5 cm on their exposed dura. After removing the weight, the paravertebral muscle and skin were closed.
The spinal cords in both control and experimental group were removed at 6 hr, 24 hr and 72 hr after contusion injury (n=3 in control group, n=5 in 6 hr, n=5 in 24 hr, n=7 in 72 hr group). Total RNA was isolated from the frozen specimens using the acid guanidinium thiocyanate-phenol-chloroform extraction method with TRIzol (Life Technoligies, Rockville, Maryland, U.S.A.). The cDNA synthesis was carried out with TaqMan Reverse Transcription reagent (Applied Biosystems) in a 20 µL reaction volume containing 4 µg total RNA, RT-PCR buffer, 500 M dNTPs, 2.5 M random hexamers 0.8 U of RNase inhibitor and 25 U of MutiScribe Reverse transcriptase. The amplifications were carried out in a 96 well plate in a 25 µL reaction volume containing 12.5 µL of 2×SYBR Green Master Mix (Applied Biosystems), 0.2 µM of each forward and reverse primer (iNOS, GAPDH) or 0.1 µM (nNOS) and 400 ng of cDNA. Oligonucleotide primer pairs for iNOS, nNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in Table 1 in detail.
The thermal profiles used for SYBR RT-PCR were 1) for iNOS: 50℃ for 2 min, 95℃ for 10 min followed by 40 cycles at 95℃ for 15 sec and at 60℃ for 1 min; and 2) for GAPDH and nNOS: at 50℃ for 2 min, at 95℃ for 10 min, followed by 40 cycles at 95℃ for 15 sec and at 62℃ for 1 min.
All experiments were performed in duplicate. Copy numbers of cDNA for iNOS and nNOS were standardized to those of GAPDH for the same sample.
Rats were sacrificed at 6 hr, 12 hr, 24 hr and 72 hr after operation. Each rat was anesthetized with pentobarbital and the spinal cord was fixed by intracardiac perfusion with 200 mL of 4% paraformaldehyde in 0.1 M phosphate-buffer. Each spinal cord tissues was serially sectioned at 2 mm interval resulting in ten segments. All sections of removed spinal cords were embedded in paraffin. Serial 10 µm cross-sections were cut and stained with hematoxylin and eosin, and selected sections were submitted to luxol fast blue staining.
TUNEL assay was performed using the same spinal cord tissues used in histologic examination. Color was developed using 3,3'-diaminobenzidine tetrachloride (DAB). Sections were treated with xylene and ethanol to remove paraffin and for dehydration. They were then washed with TBS and incubated in 3% H2O2 solution for 20 min. The sections were treated with 5 µg/mL proteinase K for 2 min at room temperature, and re-washed with phosphate buffered saline (0.1 M, pH 7.4, PBS). The sections were then treated with a TUNEL reaction mixture (terminal deoxynucleotidyl transferase, nucleotide mixture, Roche, Mannheim, Germany) at 37℃ for 1 hr, and then the sections were washed with distilled water (D/W). They were then re-incubated in anti-fluorescein antibody-conjugated with horse-radish peroxidase at room temperature for 30 min, re-washed, and then visualized using the ABC technique and 0.05% 3,3'-diamino-benzidine (DAB, Sigma) as a chromogen. The numbers of TUNEL positive cells were counted by a pathologist: at ×200 magnification, 30 fields/section. The TUNEL-positive cells were counted separately in the gray and white matter at ten axial levels. (rostral side- R0: at the epicenter, R1: at the 2 mm from the epicenter, R2: at the 4 mm from the epicenter, R3: at the 6 mm from the epicenter, and R4: at the 8 mm from the epicenter) (caudal side- C0: at the epicenter, C1: at 2 mm from the epicenter, C2: at 4 mm from the epicencer, C3: at 6 mm from the epicencer, and C4: at 8 mm from the epicenter).
Rats received i.p. 10 mg/kg of S-methylisothiourea sulfate (SMT) from Calbiochem (La Jolla, CA, U.S.A.) or 0.9% saline in the same volume. The administration of SMT was performed 15 min before and 24 hr after operation. At 6 hr (n=3), the spinal cord was removed and segments were prepared in the same way as shown above. TUNEL-positive cells were counted in the same way as shown above and we compared the TUNEL-positive cell count before and after iNOS inhibition. Statistical analysis of the TUNEL assay was performed using Mann-Whitney U-test. p<0.05 was considered significant.
To verify the inhibitory effect of SMT to NO production, we stained NO indirectly using anti-nitrotyrosine antibody (Upstate, Lake Placid, NY 12946, U.S.A.). Ten µm-thick sections of spinal cord tissues with and without SMT treatment, respectively, were treated with xylene or ethanol for paraffin removal and dehydration. Sections were boiled for 10 min with sodium citrate (pH 6.0), washed with secondary distilled water, and treated with normal serum for 40 min at room temperature to block nonspecific reactions. Anti-nitrotyrosine antibody containing 0.1% bovine serum albumin (BSA) (Bovine Albumin, Sigma) was diluted with Tris buffered saline (TBS) buffer (1:200), and then the sections were incubated at 4℃ for 12 hr. After washing in TBS buffer, the sections were re-incubated with goat anti-mouse AlexFluor 488 secondary antibody (Molecular Probe, 2333AA Leiden, Netherlands) at room temperature for 40 min and re-washed with TBS. They were then mounted with water-soluble mounting media and observed under immunofluorescence microscope.
FITC-labeled anti-iNOS monoclonal antibody (BD Transduction Laboratories, Mountain View, CA, U.S.A.) and anti-nNOS polyclonal antibody (Chemicon, Temecula, CA, U.S.A.) were used for immunofluorescence studies. Mouse monoclonal antibodies against rat microglia (ED-1, Serotec, U.K.), neuron (synaptophysin, DAKO, Denmark), oligodendrocyte (anti-oligodendrocyte monoclonal antibody, Chemicon, Temecula, CA 92590, U.S.A.), and astrocyte (GFAP, AlexFluor 594 conjugated, Molecular Probe, 2333AA Leiden, Netherlands), were used for iNOS double staining.
Segments of spinal cords plus controls were obtained for iNOS and nNOS double staining. Rats were sacrificed at 24 hr (n=1), and 72 hr (n=1) after the operation. Ten µm-thick sections of the removed spinal cords were then boiled for 10 min with sodium citrate (pH 6.0), washed with secondary distilled water, and treated with normal serum for 40 min at room temperature to block nonspecific reactions. FITC-labeled iNOS antibody (1:300) containing 0.1% BSA was diluted with TBS buffer (1:300) and then the sections were incubated in this medium at 4℃ for 12 hr. After washing with TBS buffer, the sections were mounted with water-soluble mounting media and observed under immunofluorescence microscopy. For nNOS stainging, the nNOS antibody (1:500) was incuabed at 25℃ for 1 hr. After washing with TBS buffer, the sections were re-incubated with goat anti-rabbit AlexFluor 568 secondary antibody (Molecular Probe, 2333AA Leiden, Netherlands) at room temperature for 40 min. After washing with TBS, the sections were mounted with water-soluble mounting media and observed under immunofluorescence microscope.
Rats were sacrificed at 6 hr (n=2) after the operation. Sections were pre-incubated in 10% normal goat serum for 40 min to block non-specific staining. For double staining, first, the sections were incubated with the appropriate combination of antibodies against ED-1 (1:150), synaptophysin (1:50), GFAP (1:100) and anti-oligodendrocyte mAb (1:10,000) and then the sections were stained with anti-iNOS antibody. For synaptophysin, ED-1, and oligodendrocyte staining, the sections were incubated at room temperature for 1 hr and then incubated with goat anti-mouse AlexFluor 568 for 40 min at room temperature and then washed. For GFAP staining, the sections were incubated at room temperature for 50 min and then washed. Finally, the sections were labeled with FITC-labeled iNOS antibody.
Data are summarized as mean±SD. We performed Students' t test for the statistical analysis of TUNEL positive cell count to the cord level. We also performed an one-way analysis of variance (ANOVA) for the statistical analysis of TUNEL positive cell count to iNOS inhibition. The statistical package was SPSS Win (SPSS, 1994).
Real time RT-PCR analysis was performed to investigate the temporal pattern of iNOS and nNOS induction following weight drop impact on the rat spinal cord. Fig. 1, 2 demonstrate the time course of iNOS and nNOS gene expression after traumatic insult and in sham controls, respectively. iNOS mRNA was increased at 6 hr, began to decline at 24 hr, and returned to that of sham controls 72 hr after injury. The caudal side of the spinal cord showed more prominent maximum than the rostral side. However, nNOS mRNA did not show such changes as iNOS mRNA. nNOS mRNA showed constitutive expression in both traumatic and sham operation group and showed same expression pattern in both caudal and rostral side also (Fig. 2).
Hematoxylin-eosin staining and luxol-fast blue staining of spinal cord sections showed that trauma induced a wide destruction of white and gray matter accompanied by massive hemorrhage. The gray matter tended to be damaged more severely than white matter. The gray matter showed massive hemorrhage and loss of cellular elements at the site of contusion, whereas the relatively spared white matter showed edema, myelin destruction, loss of axons and glia, and microcyst formation.
To investigate the relationship between iNOS induction and cell death, the TUNEL-positive cells were counted before and after iNOS inhibition. In the 24 hr group, the TUNEL positive cell count was 42.87±14.075 before iNOS inhibition, which it was 42.38±8.520 after iNOS inhibition. In the 72 hr group, the TUNEL positive-cell count was 195.20±27.759 before iNOS inhibition, whereas it was 29.30±11.286 after iNOS inhibition. These results suggest that after iNOS inhibition, the TUNEL positive cell count decreased significantly in the 72 hr group (p<0.05), however, the TUNEL positive cell count did not decrease in the 6 hr group (Fig. 3). To verify iNOS inhibition of SMT, anti-nitrotyrosine staining after treatment of SMT was done. Also, anti-nitrotyrosine staining revealed markedly reduced NO production after iNOS inhibition (data not shown).
Few TUNEL-positive cells were observed in sham operation group. The number of TUNEL-positive cells per section has increased until 72 hr after contusion. The number of TUNEL positive cells was larger in the 72 hr group (mean count: 123.58±10.46) than the 24 hr group (mean count: 31.10±4.89). This result suggests that cell death after trauma increases until 72 hr. In the 24 hr group, TUNEL-positive cell count did not show significant difference between the cord levels (2 mm rostral from epicenter (R1), 25.10±8.82; 4 mm rostral (R2), 28.40±10.29; 6 mm rostral (R3), 20.00±6.36; and 8 mm rostral (R4), 30.10±8.86; 2 mm caudal (C1), 79.70±27.36; 4 mm caudal (C2), 43.60±14.25; 6 mm caudal (C3), 11.70±4.98; and 8 mm caudal (C4), 10.20±3.72). In the 72 hr group, TUNEL-positive cell count showed no significant difference between the cord levels (2 mm rostral from epicenter (R1), 128.64±28.43; 4 mm rostral (R2), 122.43±34.25; 6 mm rostral (R3), 58.64±19.89; and 8 mm rostral (R4), 79.43±24.31; 2 mm caudal (C1), 176.43±34.59; 4 mm caudal (C2), 159.21±29.58; 6 mm caudal (C3), 106.36±28.52; and 8 mm caudal (C4), 103.50±26.34). In the 24 hr group, the TUNEL-positive cell count showed the same distribution pattern in both rostral (mean count: 35.90±4.22) and caudal side (mean count: 36.30±8.82). However, in the 72 hr group, the TUNEL-positive cell count was larger in the caudal side (mean count: 149.88±15.01) than rostral side (mean count: 97.29±13.82) and this result was statistically significant The number of TUNEL positive cells in gray matter was larger in the 72 hr group (mean count: 129.48±12.33) than the 24 hr group (mean count 20.13±6.31). The white matter also showed the same pattern as in gray matter and the TUNEL positive cell count was 42.08±7.15 and 117.68±16.98 in 24 hr and 72 hr group respectively. This result suggests that the apoptotic cell death occurred in both gray and white matter after SCI increased until 72 hr and there is no statistically significant difference in the rate of increase the number of TUNEL positive cell in gray and white matter (p>0.05) (Fig. 4).
We have investigated the temporal and spatial pattern of iNOS and nNOS expression in the contused spinal cord. iNOS-positive cells were not observed in the control group (Fig. 5A). They appeared at 24 hr after SCI, persisting until 72 hr after SCI (Fig. 5B). nNOS-positive cells were observed in both the control and the experimental group. The nNOS-positive cells showed aberrant neuronal expression in the time course in contrast to that of iNOS showing neuronal cell body expression. The nNOS protein was expressed in the cell body until 24 hr after SCI (Fig. 6A) and then moved to the cytoplasmic processes of neurons 72 hr after SCI (Fig. 6B).
For characterization of the iNOS-positive cells, we have performed double labeling with a combination of anti-iNOS antibody and ED-1 (Fig. 7A), synaptophysin (data not shown), GFAP (Fig. 7B) and anti-oligodendrocyte mAb (data not shown). Most of the iNOS positive cells were positive to ED-1 and synaptophysin, and they were also weakly positive to GFAP and anti-oligodendrocyte mAb. These results suggest that the iNOS may be induced by astroglial cell as well as miroglia and neurons.
In this study, we have investigated the temporal and spatial expression patterns of iNOS and nNOS after SCI in rat. According to real time RT-PCR, the iNOS mRNA peaked at 6 hr, decreased at 24 hr, and then returned to control levels 72 hr after SCI. This result is concordant with that of Satake et al. (17). However, the nNOS expression was constitutive in both the control and experimental groups. Double-labeling iNOS antibody with cell markers revealed that microglial cells, neurons, and some astroglial cells expressed iNOS protein in their cytoplasms after SCI. According to this study, all the cell types present in the traumatized spinal cord seemed to play a role in producing NO via iNOS, and this result does not coincide with the results of Wada et al. (18, 19). They reported that after traumatic brain injury, the major cellular sources of iNOS are the astrocytes and macrophages. Immunofluorescence analysis revealed an aberrant neuronal expression of nNOS during the time course. We first demonstrated the aberrant spatial expression of nNOS in neurons following SCI. However, the reason for this result remain unclear. nNOS is actually protective against cell death and it is constantly expressed in neurons, yet the aberrant neuronal expression of nNOS may result in the loss of its neuroprotective role. Further study is needed to prove this proposal. iNOS is a known inducer of the extrinsic apoptotic pathway that brings about caspase-3 activation and affects apoptosis. Therefore, we observed the effect of iNOS on neuronal and glial apoptosis, and this led us to conclude that the neurologic deficit may continue for up to 72 hr after trauma because iNOS mRNA was observed for up to 72 hr. In addition, the neurologic deficit progresses in both directions and it may be more detrimental in the caudal side than in the rostral side at 72 hr after SCI. This was shown by the findings where the TUNEL positive cell count was higher in the caudal side than in the rostral side. SMT was injected intraperitoneally at 6 hr and 72 hr after SCI, because the iNOS mRNA peaked at 6 hr after SCI, and the apoptosis peaked at 72 hr after SCI. After selective iNOS inhibition was induced, the apoptosis was reduced in the 72 hr group, but not in the 6 hr group. We can suppose that until 72 hr, other factors instead of iNOS may induce apoptosis, and iNOS, may induce apoptosis after 72 hr following SCI. We also verified that iNOS is involved in delayed neuronal and glial cell death, in part via the apoptotic signaling in SCI. Therefore, the inhibition of the iNOS activity may have a beneficial effect on reducing the acute secondary SCI, and the iNOS inhibition should be done before 6 hr post-SCI.
By employing luxol fast blue staining, the destructive changes in the epicenter were observed to be more severe in the gray matter than in the white matter. This might be due to the softer consistency and the greater vascularity of the gray matter as has been mentioned previously by Wolman (20).
Figures and Tables
 | Fig. 1iNOS mRNA expression by real time reverse transcription-polymerase chain reaction (RT-PCR) in the control group and 6 hr, 24 hr, 72 hr after contusion injury. The relative amounts of iNOS mRNA shows noticeable changes with time. At 6 hr post-SCI, the increase in the level of iNOS mRNA was prominent, especially in the caudal side. |
 | Fig. 2nNOS mRNA expression by real time RT-PCR in the control group and at 6 hr, 24 hr, and 72 hr after contusion injury. The relative amounts of nNOS mRNA showed no noticeable change with time, and were much the same in the control and experimental groups. |
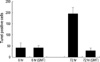 | Fig. 3The terminal deoxynucleotidyl-transferase-mediated dUDP-biotin nick end-labeling (TUNEL)-positive cell count was reduced after iNOS inhibition 72 hr after spinal cord injury (p<0.05). 6 hr=6 hr after SCI before iNOS inhibition, 6 hr (SMT)=6 hr after SCI after iNOS inhibition, 72 hr=72 hr after SCI before iNOS inhibition, 72 hr (SMT)=72 hr after SCI after iNOS inhibition. |
 | Fig. 4The terminal deoxynucleotidyl-transferase-mediated dUDP-biotin nick end-labeling (TUNEL)-positive cell count was higher at 72 hr post-SCI than at 24 hr post-SCI and higher in the caudal side than in the rostral side. No statistical significance could be attached to different of TUNEL-positive cell count in the gray matter and white matter (p<0.05). |
 | Fig. 5Immunofluorescence of iNOS in the rat spinal cord of the control (A, ×200), and in the 72 hr group (B, ×200). In the control group, iNOS activity is absent. In the experimental group, iNOS activity appeared at 24 hr and increased in intensity at 72 hr. |
 | Fig. 6Immunofluorescence of nNOS in the rat spinal cord of the control (A, ×200), and of the 72 hr group (B, ×200), 72 hr after spinal cord contusion. nNOS activity was observed in both the control and experimental groups. In the experimental group, nNOS immunoreactivity is observed in the neuronal cell body 24 hr post-SCI and in the cell processes at 72 hr post-SCI. |
References
1. Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, Part I: Pathophysiologic mechanisms. Clin Neurophamacol. 2001. 24:254–264.

2. Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995. 5:407–413.

3. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991. 75:15–26.

4. Demopoulos HB, Flamm ES, Seligman ML, Mitamura JA, Ransohoff J. Popp AJ, editor. Membrane perturbations in central nervous system injury: Theoretical basis for free radical damage and a review of the experimental data. Neural Trauma. 1979. New York: Raven Press;63–78.
5. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001. 26:24 Suppl. S2–S12.

6. Freeman RS, Estus S, Johnson EM Jr. Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron. 1994. 12:343–355.

7. Rotello RJ, Fernandez PA, Yuan J. Anti-apogens and anti-engulfens: monoclonal antibodies reveal specific antigens on apoptotic and engulfment cells during chicken embryonic development. Development. 1994. 120:1421–1431.

8. Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997. 3:73–76.

9. Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998. 89:911–920.

10. Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997. 50:798–808.

11. Matsuyama Y, Sato K, Kamiya M, Yano J, Iwata H, Isobe K. Nitric oxide: a possible etiologic factor in spinal cord cavitation. J Spinal Disord. 1998. 11:248–252.
12. Dawson VL, Kizushi VM, Huang PL, Snyder SH, Dawson TM. Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci. 1996. 16:2479–2487.

13. Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, Fukuzawa K. Roles of nitric oxide in compression injury of rat spinal cord. Free Radic Biol Med. 1996. 20:1–9.
14. Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997. 28:1283–1288.

15. Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitrosocompounds. Nature. 1993. 364:626–632.

16. Allen A. Surgery of experimental lesions of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. JAMA. 1911. 57:878–880.
17. Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res. 2000. 85:114–122.

18. Wada K, Chatzipanteli K, Kraydieh S, Busto R, Dietrich WD. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery. 1998. 43:1427–1436.

19. Wada K, Chatzipanteli K, Busto R, Dietrich WD. Effects of L-NAME and 7-NI on NOS catalytic activity and behavioral outcome after traumatic brain in the rat. J Neurotrauma. 1999. 16:203–212.
20. Wolman L. The disturbances of circulation in traumatic paraplegia in acute and late stages: a pathological study. Paraplegia. 1965. 59:213–226.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download