Abstract
Latent transforming growth factor (TGF)-β-binding protein (LTBP) is required for the assembly, secretion, matrix association, and activation of latent TGF-β complex. To elucidate the cell specific expression of the genes of LTBP-1 and their splice variants and the factors that regulate the gene expression, we cultured primary human glomerular endothelial cells (HGEC) under different conditions. Basal expression of LTBP-1 mRNA was suppressed in HGEC compared to WI-38 human embryonic lung fibroblasts. High glucose, H2O2, and TGF-β1 upregulated and vascular endothelial growth factor (VEGF) further downregulated LTBP-1 mRNA in HGEC. RT-PCR with a primer set for LTBP-1S produced many clones but no clone was gained with a primer set for LTBP-1L. Of 12 clones selected randomly, Sca I mapping and DNA sequencing revealed that only one was LTBP-1S and all the others were LTBP-1SΔ53. TGF-β1, but not high glucose, H2O2 or VEGF, tended to increase LTBP-1SΔ53 mRNA. In conclusion, HGEC express LTBP-1 mRNA which is suppressed at basal state but upregulated by high glucose, H2O2, and TGF-β1 and downregulated by VEGF. Major splice variant of LTBP-1 in HGEC was LTBP-1SΔ53. Modification of LTBP-1SΔ53 gene in HGEC may abrogate fibrotic action of TGF-β1 but this requires confirmation.
Transforming growth factor (TGF)-β is usually secreted as a biologically inactive large latent TGF-β complex (1-3). The latent form of TGF-β is composed of three elements, i.e., the mature TGF-β, the latency-associated propeptide (LAP) and the latent TGF-β-binding protein (LTBP) (2, 4-6).
During secretion, TGF-β is cleaved from its carboxy-terminal LAP (7), which remains non-covalently bound to the mature TGF-β conferring latency to the complex (8). In the large latent complex TGF-β is attached by disulfide bonding of its LAP-part to a high molecular weight LTBP (9, 10). LTBP was first cloned in 1990 (6) and later renamed LTBP-1. Three other LTBP isoforms (LTBPs-2, -3 and -4) have been cloned since.
LTBP is required for the assembly and secretion of latent TGF-β (11) and also contribute to the matrix association (12) and activation (13) of latent TGF-β complex. The major fraction (>90%) of secreted LTBP does not contain TGF-β (3, 11, 12) and thus LTBP evidently possesses separate roles in vivo as structural proteins of extracellular matrix (ECM) and as TGF-β targeting molecules (12, 14). Human LTBPs-1, -2, and -4 have more than one alternative N-terminal region (5, 6, 15, 16). LTBP-1 appears as two mRNA species in Northern blots. These mRNAs encode for two different N-terminal variants, the longer LTBP-1L and the shorter LTBP-1S isoforms (5, 6). Independent promoters regulate the expression of LTBP-1L and LTBP-1S in a cell type-specific manner (17). LTBP-1L was found to interact more efficiently with ECM (16) suggesting the importance of the N-termini of LTBP in the ECM association. LTBP-1L is mainly expressed in heart, placenta, kidney, and prostate, whereas LTBP-1S has a wider expression pattern and appears also in the lung, skeletal muscle, testis, and ovary (16). The existence of four LTBP isoforms, their various alternatively spliced forms, and diverse mRNA expression patterns in different tissues and developmental stages suggest a substantial variety of functional properties for LTBP.
Human liver myofibroblasts express transcripts of all three TGF-β isoforms, all four LTBP isoforms, and nearly all splice variants of LTBP-1 and -4 suggesting that LTBP modulates the bioactivity of TGF-β in the diseased liver (18). LTBP-1 expression is co-regulated with TGF-β1 (11, 12, 19). LTBP-1 expression is under the regulation of retinoic acid and corticoids in normal and SV-40 virus-transformed human lung fibroblasts (19). Basal expression levels of LTBP-1 were significantly reduced in the transformed cells. TGF-β1 increased its own expression as well as LTBP-1 levels in both cell lines but the response was clearly of lower magnitude in transformed cells (19).
Anomalous expression of LTBP may be associated with human diseases. Overexpression of LTBP-1 was observed in IgA nephropathy (20), chronic C hepatitis (21), idiopathic pulmonary fibrosis (22), human capsular opacification (23), and ovarian carcinoma (24).
TGF-β1 is a potent regulator of ECM synthesis and degradation and is the key mediator of tissue fibrosis (25). Progressive fibrosis in the kidney, liver, lung, heart, bone marrow, skin, and lens is both the major cause of suffering and death and an enormous burden to cost of health care (25). Renal ablation induces glomerular endothelial as well as mesangial cells to express TGF-β1 and matrix protein mRNAs leading to glomerular fibrosis (26). TGF-β1 expression is increased in diabetic kidney in experimental animals (27, 28) and in humans (28). High glucose stimulates TGF-β1 mRNA and protein expression in cultured glomerular mesangial cells (29), glomerular epithelial cells (30), and proximal tubular epithelial cells (31). High glucose-induced fibronectin upregulation in glomerular mesangial cells is mediated by TGF-β1 (29).
Blocking the secretion and activation of latent TGF-β complex through modification of LTBP gene may be one way of preventing TGF-β1-induced tissue fibrosis. Little is known, however, about the regulation of LTBP gene expression or differential expression of LTBP isoforms and their alternatively spliced forms in the kidney.
We, therefore, examined the basal expression of LTBP-1 and factors that regulate the expression of LTBP-1 transcripts and their splice variants in human glomerular endothelial cells.
Taq DNA polymerase, other restriction enzymes and dual luciferase assay system were purchased from Promega (Promega Corporation, Madison, WI, U.S.A.).
HGEC was isolated from the kidney removed from a Korean patient with renal carcinoma. An informed consent was obtained from the patient for experimental use of the kidney tissue. Isolation, culture, and characterization of HGEC were carried out according to the methods of Green et al. (32) and Park et al. (33). In brief, cortex from apparently normal part of the kidney was excised just after removal of the kidney and then glomeruli were isolated using sieves. Glomeruli isolated were incubated at 37℃ in Dulbecco's modified Eagle's medium (DMEM GIBCO BRL; Grand Island, NY, U.S.A.) containing 20% of fetal bovine serum (FBS GIBCO BRL), penicillin G (100 U/mL GIBCO BRL) and streptomycin (100 µg/mL GIBCO BRL). Cells from out growth of glomeruli showing cobblestone morphology and capillary-like tubule formation were selected as candidates for HGEC using cloning cylinder. The colonies were amplified in 60-mm culture dish with the same media. Immunofluoresence staining for Factor VIII was carried out for the confirmation of HGEC. Cells from passage 4-7 were used for this study. To analyze the expression of LTBP-1, the cells were cultured in DMEM containing 20% of FBS, penicillin G (100 U/mL) and streptomycin (100 µg/mL). Before each experiment, the cells were synchronized by serum-starvation in DMEM without FBS for 24 hr. The cells were treated with DMEM containing 30 mM glucose (high glucose), 100 µM H2O2, 2.5 ng/mL of TGF-β1 (R&D System, Minneapolis, MN, U.S.A.) or 10 ng/mL of VEGF (R&D System) and incubated for 12 hr or 24 hr. Cells treated with DMEM containing 5.6 mM glucose served as control. Cells were also treated with mannitol 25 mM added to media containing 5.6 mM glucose as an osmotic control. The dosage of glucose, H2O2, TGF-β, and VEGF used in this study was based upon published reports from our own laboratory and others.
To evaluate the total expression level of LTBP-1 mRNAs including LTBP-1L, LTBP-1S and LTBP-1SΔ53, RT-PCR was carried out with primers (LSF3 and LSR1 in Table 1) whose sequences are found commonly at the 3'end of all LTBP-1 mRNAs (Fig. 1). PCR was carried out with an initial denaturation at 95℃ for 3 min and then cycled 30 or 40 times for one min at 95℃, followed by one min at 60℃ and 2 min at 72℃. Then the reaction was maintained at 72℃ for an additional 5 min before completion. As a control, human β-actin was amplified by RT-PCR with primers (HBAF and HBAR in Table 1) under the same condition. In addition, Northern blot analysis was carried out to confirm the RT-PCR result. The PCR product (1,040 bp) was labeled with [α-32P] dCTP and used as probe.
To compare the expression level of LTBP-1S of HGEC with that of WI-38 lung fibroblasts (American Type Culture Collection Rockville, MD, U.S.A.), transient transfection assay was carried out with luciferase reporter construct, L-1S PROM (a gift of Dr. Keski-Oja, 17). HGEC and WI-38 cells were cultured in 60-mm diameter culture plates. At 60% confluency, cells were co-transfected with 3 µg L-1S PROM and pRL-CMV control plasmid (Promega) containing Renilla luciferase gene using GenePORTER2 transfection reagent according to the manufacturer's instruction (Gene Therapy System, Telesis Court, San Diego, CA, U.S.A.). Four hours after transfection, the media were changed with the same fresh media, and incubated for another 24 hr. The cells were washed twice with phosphate-buffered saline (PBS) and lysed with 500 µL of Passive Lysis Buffer in Dual Luciferase kit (Promega Corp., Madison, WI, U.S.A.). The luciferase activity of the lysate was measured by using TD-20/20 luminometer (Tunner Designs, Sunnyvale, CA, U.S.A.).
Total RNA was isolated from cultured HGEC or WI-38 with TRI RAGENT (Molecular Research Center, Cincinati, OH, U.S.A.), and the first stranded cDNA was synthesized by AMV reverse transcriptase (Promega Corporation, Madison, WI, U.S.A.) using oligo dT15 primer with 3 µg of total RNA. For the amplification of the LTBP-1 genes, two different sets of PCR primers (LF1/LSR1 and SF1/LSR1) were designed according to the DNA sequences from GenBank (M34057 and L48925) as shown in Table 1. For the PCR amplification of LTBP-1L and LTBP-1S, 20 pmol of each primer set was used. PCR was carried out with an initial denaturation at 95℃ for 3 min and then was cycled 30 times for one min at 95℃, followed by one min at 50℃ and 8 min at 72℃. Then the reaction was maintained at 72℃ for an additional 15 min before completion. The PCR products were cloned directly on PCR 2.1-TOPO TA cloning vector (Invitrogen Corp., Carlsbad, CA, U.S.A.). To confirm that the PCR product was LTBP-1 gene, nucleotide sequences of the clones were determined using a BigDyeTM-terminator kit (Perkin-Elmer, Wellesley, MA, U.S.A.) and analyzed with ABI 310 genetic analyzer (Applied Biosystem Inc, Foster City, CA, U.S.A.).
To distinguish the mRNA of LTBP-1SΔ53 from that of LTBP-1S, RT-PCR was carried out with internal primers (LSF2 and LSR2 in Table 1) prepared according to the sequences found near up-stream and down-stream of the deleted region of LTBP-1SΔ53. With the primer set, LTBP-1S mRNA will give 469 bp PCR product while 310 bp PCR product will be amplified from LTBP-1SΔ53. PCR was carried out with an initial denaturation at 95℃ for 3 min and then was cycled 30 or 40 times for one min at 95℃, followed by one min at 55℃ and 2 min at 72℃. Then the reaction was maintained at 72℃ for an additional 5 min before completion. One fifth of the PCR product was electrophoresed on 1.5% agarose gel.
All results are expressed as mean±standard error (SE). Analysis of variance (ANOVA) was used to assess the difference between multiple groups. If the F statistics was significant, the mean values obtained from each group were then compared by Fisher's least significant difference method. P value <0.05 was used as the criterion for a statistically significant difference.
To evaluate the cell type-specific expression of LTBP-1, RT-PCR was carried out with total RNA from cultured HGEC and WI-38 lung fibroblasts. PCR product was easily detected on the agarose gel after 30-cycle reaction with total RNA of WI-38. Similar amount of PCR product from total RNA of HGEC was recognized when the PCR was performed 40 cycles. The expression level of LTBP-1 in HGEC was much lower than that in WI-38. The RT-PCR result was supported by Northern blot analysis as well as by transient transfection assay. As shown in Fig. 2A, two LTBP-1 transcripts (7.0 kb for LTBP-1L and 5.2 kb for LTBP-1S) were detected from total RNA of WI-38, whereas no signals were obtained from HGEC. In addition, when L-1S PROM was introduced into cells, luciferase was hardly expressed in HGEC but very strong luciferase activity was measured in the extract of WI-38 (Fig. 2B).
As shown in Fig. 3, high glucose, H2O2, and TGF-β1 increased LTBP-1 mRNA expression by 1.2 (±0.2), 1.6 (±0.3) and 1.3 (±0.2) folds that of control, respectively, at 12 hr after treatment. High glucose and TGF-β1 but not H2O2, further increased LTBP-1 expression to 1.5 (±0.3) and 1.8 (±0.3) folds, respectively, at 24 hr. Mannitol 25 mM added to media containing 5.6 mM glucose failed to increase LTBP-1 mRNA (data not shown). On the contrary, VEGF suppressed LTBP-1 expression to 80% of control at 12 hr and to 60% at 24 hr.
To obtain the respective LTBP-1L and LTBP-1S clone, two different PCR primer sets (LF1/LSR1and SF1/LSR1) were used for RT-PCR and the PCR products were analyzed on 1% agarose gel electrophoresis. In the case of LTBP-1S, about 4.2 kb DNA fragment was observed whereas there was no detectable PCR product for LTBP-1L (data not shown). The PCR products were cloned by using PCR 2.1-TOPO TA cloning system, and then introduced into E. coli Top10F'. After transformation, the bacterial cells were cultured on LB agar plate supplemented with 100 µg/mL of ampicillin and 5-bromo-4-chloro-3-indolyl-β-galactopyranoside. Some white colonies were grown on the plate when PCR product of LTBP-1S was applied. However, no transformant was grown on the same agar media when PCR product of LTBP-1L was employed. Twenty recombinant plasmids were isolated randomly, and PCR was carried out with the same primers (SF1/LSR1) to identify the insert on the cloning vector. Twelve recombinant plasmids tested yielded the 4.2 kb PCR products and nucleotide sequences of the two clones selected randomly were determined. The DNA sequences revealed that both clones were LTBP-1SΔ53, an alternative spliced-form of LTBP-1S.
To confirm that the other 10 clones were either LTBP-1S or LTBP-1SΔ53, the recombinant plasmids obtained were digested with Sca I. The Sca I site is unique in LTBP-1S and does not exist in LTBP-1SΔ53 since the restriction site is located in the deleted region of LTBP-1SΔ53. PCR 2.1-TOPO TA cloning vector also possesses one Sca I site, therefore, LTBP-1S clone possesses two Sca I sites whereas LTBP-1SΔ53 has only one. As shown in Fig. 4, only one clone was a candidate for LTBP-1S (lane 12) and the others were all for LTBP-1SΔ53. The candidate clone for LTBP-1S was confirmed as LTBP-1S by determination of its DNA sequence. The DNA and deduced amino acid sequences of newly cloned LTBP-1S deposited to GenBank (accession No. AF489528) were compared to LTBP-1S sequence from GenBank data bases (accession No. M34057). The new LTBP-1S clone from Korea has four different nucleotides (1093T→C; 1510G→A; 3498T→G; 4000T→G), and three of them change amino acid (365 Tyr→His; 604 Val→Thr; 1334 Phe→Val). The first nucleotide of start codon is 1, and the nucleotides showing after numbers are found in the sequences of M34057.
To confirm that LTBP-1SΔ53 is the major splice variant of LTBP-1 transcript in HGEC, the transcript was analyzed by RT-PCR with internal primer set (LSF2 and LSR2). The expecting size of RT-PCR product was 469 bp for LTBP-1S and 310 bp for LTBP-1SΔ53. As a control, total RNA from WI-38 was subjected to RT-PCR with the same condition. Three different-sized PCR products, 310 bp, 469 bp and an unexpected 620 bp were produced from total RNA of WI-38 (Fig. 5). Two smaller products were eluted from agarose gel and cloned on PCR 2.1-TOPO TA cloning vector. DNA sequences were determined and those DNA fragments were confirmed as a part of LTBP-1SΔ53 and LTBP-1S as expected (data not shown). The splicing pattern of LTBP-1 was different between WI-38 and HGEC. In the case of WI-38, 469 bp PCR product was found as the major form. In HGEC, however, 310 bp PCR product was the major form and all PCR product was LTBP-1SΔ53 in some case (Fig. 5). The results from cDNA cloning, Sca I mapping, and RT-PCR with internal primers indicated that the major splice variant of LTBP-1 is LTBP-1SΔ53 in HGEC.
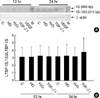
As shown in Fig. 6, high glucose or H2O2 did not change the ratio of LTBP-1SΔ53/LTBP-1S at 12 and 24 after treatment. TGF-β1 increased the LTBP-1SΔ53 mRNA and the LTBP-1SΔ53/LTBP-1S ratio at 24 hr although the difference did not reach statistical significance.
Chronic progressive renal disease is characterized by glomerular and tubulointerstitial fibrosis. TGF-β1 is a potent regulator of ECM synthesis and degradation and is the key mediator of renal fibrosis (25).
Renal ablation, an animal model of chronic renal failure, induces glomerular endothelial as well as mesangial cells to express TGF-β1 and ECM mRNAs leading to glomerular fibrosis or glomerulosclerosis (26).
Diabetic nephropathy, the single most common cause of endstage renal disease, is also characterized by overexpression of TGF-β1 and excessive accumulation of ECM in the kidney. High glucose upregulates TGF-β1 and ECM genes and proteins in glomerular mesangial and tubular epithelial cells and in diabetic kidney (34). Antioxidants inhibit high glucose-induced TGF-β1 and ECM expression in renal cells and ameliorate features of diabetic nephropathy suggesting that reactive oxygen species mediate high glucose-induced TGF-β1 and ECM expression (34).
Modification of LTBP gene may prevent secretion and activation of latent TGF-β complex and abrogate fibrotic action of TGF-β1. Little is known, however, about the regulation of LTBP expression or differential expression of LTBP isoforms and their splice variants in the kidney.
We, therefore, examined the basal expression of LTBP-1 transcripts and their splice variants in HGEC and the factors that regulate LTBP-1 gene expression in HGEC. We found that basal expression of LTBP-1 mRNA was suppressed in HGEC as compared to WI-38, human embryonic lung fibroblasts, that high glucose, H2O2, and TGF-β1 upregulated and VEGF downregulated LTBP-1 expression, and that the major splice variant of LTBP-1 was LTBP-1SΔ53. TGF-β1, but not high glucose or H2O2, had a tendency to increase LTBP-1SΔ53 mRNA.
The results from RT-PCR and transfection assay strongly suggested that the basal expression of LTBP-1 is suppressed in HGEC. LTBP-1S expression was less than 4% that of WI-38 cells. It is possible that this suppression is related to the background of HGEC used in this study. The cells were obtained from a patient with renal cell carcinoma. Basal expression of LTBP-1 was significantly reduced in SV-40 virus-transformed human lung fibroblasts compared to normal lung fibroblasts (19). TGF-β1 increased LTBP-1 levels in both cell lines, but the response was clearly of lower magnitude in transformed cells (19). This may reflect insensitivity to TGF-β1, which is a common feature for malignant cells. The mRNA level of LTBP-1 was decreased in neoplastic parenchymal cells but increased in the ECM of hepatocellular and fibrolamellar carcinoma of the liver (35). Since TGF-β1 is known to be overexpressed in liver tumors, the results suggest enhanced synthesis of TGF-β1 without binding to LTBP-1 resulting in the decreased bioavailability of TGF-β1 in tumor cells. On the other hand, this suppressed expression of LTBP-1 mRNA may be a characteristic of glomerular endothelial cells. This needs to be confirmed in glomerular endothelial cells isolated from normal human kidney.
We found that high glucose, H2O2, and TGF-β1 upregulated and VEGF downregulated LTBP-1 mRNA in HGEC. High glucose and H2O2 upregulate TGF-β1 mRNA levels in renal cells (34). TGF-β1 auto-regulates its own expression (25). LTBP-1 plays a central role in secretion and activation of latent TGF-β complex and LTBP-1 expression is co-regulated with TGF-β1 (11, 12, 19). TGF-β1 upregulated mRNA levels of both TGF-β1 and LTBP-1 in normal and transformed human lung fibroblasts (19). The expression of LTBP-1 mRNA in lung fibroblasts was also upregulated by retinoic acid and corticoids (19). The significance of downregulation of LTBP-1 mRNA by VEGF in HGEC is not clear at this time. Glomerular capillary endothelial cells express VEGF receptor (36) and proliferate in response to VEGF. TGF-β induces VEGF expression in lung fibroblasts (37) and VEGF attenuates actions of TGF-β1 in human endothelial cells (38). It is possible that VEGF acts as an adaptive mechanism to TGF-β-induced upregulation of LTBP-1.
The present data suggest that the major form of splice variant of LTBP-1 in HGEC is LTBP-1SΔ53, a splice variant of LTBP-1S, unlike in WI-38 lung fibroblasts where both LTBP-1L and -1S were found. In SV-40 virus-transformed WI-38 lung fibroblasts a regulatory element repressed the transcription of LTBP-1S by a cell specific manner (17).
TGF-β1, but not high glucose or H2O2, had a tendency to upregulate LTBP-1SΔ53 mRNA and the LTBP-1SΔ53/LTBP-1S ratio in HGEC.
In conclusion, HGEC express LTBP-1 mRNA which is suppressed at basal state but upregulated by high glucose, H2O2, and TGF-β1 and further downregulated by VEGF. The major splice variant of LTBP-1 in HGEC was LTBP-1SΔ53 and the mRNA was upregulated by TGF-β1. It is tempting to speculate that modification of LTBP-1SΔ53 gene in HGEC may abrogate fibrotic action of TGF-β1. Future studies are needed to demonstrate the effect of deletion or mutation of LTBP-1SΔ53 gene in HGEC on TGF-β1 expression and the fibrotic action of TGF-β1.
Figures and Tables
Fig. 1
Structural features of LTBP-1S and LTBP-1SΔ53. Simple structural features of LTBP-1S, primer sets, the size of PCR products and the deleted region in LTBP-1SΔ53 are shown. A putative heparin-binding site is shown by amino acid sequence (HRRRPIHHHVGK) and other typical motifs, repeats and sites are illustrated with various symbols.

Fig. 2
Estimation of basal LTBP-1 expression in HGEC. Northern blot analysis for LTBP-1 expression was carried out with RNA from HGEC and WI-38. The results of three independent experiments are presented. 28S and 18S served as internal controls (A). (B) Transient transfection assay for LTBP-1 expression was carried out with L-1S PROM, a reporter construct covering the 1,751 bp upstream region of LTBP-1S gene. The reporter construct was introduced with pRL-CMV into HGEC and WI-38 and cell lysate of each culture was prepared after 24 hr of transfection. The mean value of the activity from three independent experiments is shown with the value of WI-38 as 100%.
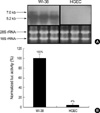
Fig. 3
Expression patterns of LTBP-1 in HGEC cultured under different conditions. The first stranded cDNA was synthesized with 3 µg of total RNA from HGEC and then PCR with primers (LSF3 and LSR1) was carried out 40 cycles. For standardization of the amount of the respective RNA applied, β-actin was amplified with the same cDNA prepared. One fifth of PCR volume was applied on the 1.5% agarose gel electrophoresis. The results of five independent experiments are shown as fold change relative to control (B). Upper panel (A) is a representative bands of PCR products from 5 experiments. C: control; HG: high glucose (30 mM); H2O2: 100 µM H2O2; TGF-β1: 2.5 ng/mL of TGF-β1; VEGF: 10 ng/mL of VEGF; M: molecular size marker; *p<0.05 compared to 12 hr control.
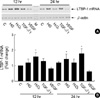
Fig. 4
Identification of LTBP-1S and LTBP-1SΔ5 by restriction mapping. Restriction mapping with Sca I (Promega) was carried out to screen the candidate clones for LTBP-1S. Two Sca I sites are in LTBP-1S (lane 12), but only one site in LTBP-1SΔ53 (lane 1-11).

Fig. 5
Alternative spliced-form of LTBP-1 RNA in HGEC and WI-38. The first stranded cDNA was synthesized with 3 µg of total RNA from HGEC and WI-38. PCR with internal primers (LSF2 and LSR2) was carried out 30 cycles for WI-38 (lane 1) and 40 cycles for HGEC (lane 2 and 3). For standardization of the amount of respective RNA applied, β-actin was amplified with 30 cycles of PCR with the same cDNA prepared. One fifth of PCR volume was applied on the 1.5% agarose gel electrophoresis.
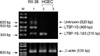
Fig. 6
Changes of the splicing pattern in HGEC cultured under different conditions. RT-PCR was done with the same method described in Fig. 5. Upper panel (A) is a representative bands for LTBP-1S and LTBP-1SΔ53 under different stimuli from PCR products from 5 independent experiments. Lower panel (B) shows the mean±SE of LTBP-1SΔ53/LTBP-1S ratio from 5 experiments. C: control; HG: high (30 mM) glucose; H2O2: 100 µM H2O2; TGF-β1: 2.5 ng/mL of TGF-β1; VEGF: 10 ng/mL of VEGF; M: molecular size marker.
References
1. Miyazono K, Hellman U, Wernstedt C, Heldin CH. Latent high molecular weight complex of transforming growth factor-β1. Purification from human platelets and structural characterization. J Biol Chem. 1988. 263:6407–6415.
2. Olofsson A, Miyazono K, Kanzaki T, Colosetti P, Engstrom U, Heldin CH. Transforming growth factor-β1, -β2, and -β3 secreted by a human glioblastoma cell line. Identification of small and different forms of large latent complexes. J Biol Chem. 1992. 267:19482–19488.
3. Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-β1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995. 270:4689–4696.

4. Wakefield LM, Smith DM, Flanders KC, Sporn MB. Latent transforming growth factor-β from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988. 263:7646–7654.
5. Kanzaki T, Olofsson A, Moren A, Wernstedt C, Hellman U, Miyazono K, Claesson-Welsh L, Heldin CH. TGF-β1 binding protein: a component of the large latent complex of TGF-β1 with multiple repeat sequences. Cell. 1990. 61:1051–1061.

6. Tsuji T, Okada F, Yamaguchi K, Nakamura T. Molecular cloning of the large subunit of transforming growth factor type β masking protein and expression of the mRNA in various rat tissues. Proc Natl Acad Sci USA. 1990. 87:8835–8839.
7. Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor-β1 precursor by human furin convertase. J Biol Chem. 1995. 270:618–624.
8. Gentry LE, Lioubin MN, Purchio AF, Marquardt H. Molecular events in the processing of recombinant type I pre-pro-transforming growth factor β to the mature polypeptide. Mol Cell Biol. 1988. 8:4162–4168.
9. Saharinen J, Taipale J, Keski-Oja J. Association of the small latent transforming growth factor-β with an eight cysteine repeat of its binding protein LTBP-1. EMBO J. 1996. 15:245–253.
10. Gleizes PE, Beavis RC, Mazzieri R, Shen B, Rifkin DB. Identification and characterization of an eight-cysteine repeat of the latent transforming growth factor-β binding protein-1 that mediates bonding to the latent transforming growth factor-β1. J Biol Chem. 1996. 271:29891–29896.

11. Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-β1-binding protein in the assembly and secretion of TGF-β1. EMBO J. 1991. 10:1091–1101.
12. Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-β associates to fibroblast extracellular matrix via latent TGF-β1 binding protein. J Cell Biol. 1994. 124:171–181.
13. Flaumenhaft R, Abe M, Sato Y, Miyazono K, Harpel J, Heldin CH, Rifkin DB. Role of the latent TGF-β binding protein in the activation of latent TGF-β by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993. 120:995–1002.
14. Dallas SL, Miyazono K, Skerry TM, Mundy GR, Bonewald LF. Dual role for the latent transforming growth factor-β binding protein in storage of latent TGF-β in the extracellular matrix and as a structural matrix protein. J Cell Biol. 1995. 131:539–549.
15. Moren A, Olofsson A, Stenman G, Sahlin P, Kanzaki T, Clasesson-Welsh L, ten Dijke P, Miyazono K, Heldin CH. Identification and characterization of LTBP-2, a novel latent transforming growth factor-β-binding protein. J Biol Chem. 1994. 269:32469–32478.
16. Olofsson A, Ichijo H, Moren A, ten Dijke P, Miyazono K, Heldin CH. Efficient association of an amino-terminally extended form of human latent transforming growth factor-β binding protein with the extracellular matrix. J Biol Chem. 1995. 270:31294–31297.

17. Koski C, Saharinen J, Keski-Oja J. Independent promoters regulate the expression of two amino terminally distinct forms of latent transforming growth factor-β binding protein-1 (LTBP-1) in a cell type-specific manner. J Biol Chem. 1999. 274:32619–32630.

18. Mangasser-Stephan K, Gartung C, Lahme B, Gressner AM. Expression of isoforms and splice variants of the latent transforming growth factor beta binding protein (LTBP) in cultured human liver myofibroblasts. Liver. 2001. 21:105–113.
19. Weikkolainen K, Keski-Oja J, Koli K. Expression of latent TGF-beta binding protein LTBP-1 is hormonally regulated in normal and transformed human lung fibroblasts. Growth Factors. 2003. 21:51–60.
20. Wada T, Hamakawa S, Hori Y, Kaname S, Shimizu S, Kurokawa K, Katoh T. Immunohistochemical localization of latent transforming growth factor-β binding protein in IgA nephropathy. Kidney Int. 1997. 52:S182–S184.
21. Kinnman N, Andersson U, Hultcrantz R. In situ expression of transforming growth factor-beta1-3, latent transforming growth factor-beta binding protein and tumor necrosis factor-alpha in liver tissue from patients with chronic hepatitis C. Scand J Gastroenterol. 2000. 35:1294–1300.
22. Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001. 56:907–915.
23. Saika S, Miyamoto T, Tanaka T, Ishida I, Ohnishi Y, Ooshima A. Latent TGF-beta binding protein-1 and fibrillin-1 in human capsular opacification and in cultured lens epithelial cells. Br J Ophthalmol. 2001. 85:1362–1366.

24. Higashi T, Sasagawa T, Inoue M, Oka R, Shuangying L, Saijoh K. Overexpression of latent transforming growth factor-beta 1 (TGF-beta 1) binding protein 1 (LTBP-1) in association with TGF-beta 1 in ovarian carcinoma. Jpn J Cancer Res. 2001. 92:506–515.
25. Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994. 331:1286–1292.

26. Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest. 1995. 96:953–964.

27. Lee HB, Cha MK, Song KI, Kim JH, Lee EA, Kim SI, Kim J, Yoo MH. Pathogenic role of advanced glycosylation end products in diabetic nephropathy. Kidney Int. 1997. 60:S60–S65.
28. Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993. 90:1814–1818.

29. Oh JH, Ha H, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-β1 and fibronectin synthesis. Kidney Int. 1998. 54:1872–1878.

30. van Det NF, Verhagen NA, Tamsma JT, Berden JH, Bruijn JA, Daha MR, van der Woude FJ. Regulation of glomerular epithelial cell production of fibronectin and transforming growth factor-β by high glucose, not by angiotensin II. Diabetes. 1997. 46:834–840.

31. Rocco M, Chen Y, Goldfarb S, Ziyadeh FN. Elevated glucose stimulates TGF-β gene expression and bioactivity in proximal tubule. Kidney Int. 1992. 41:107–114.

32. Green DF, Hwang KH, Ryan US, Bourgoignie JJ. Culture of endothelial cells from baboon and human glomeruli. Kidney Int. 1992. 41:1506–1516.

33. Park S, Ahn H, Kim SW, Lee JD, Park JS. Culture of human glomerular endothelial cells. Korean J Nephrol. 1997. 16:221–229.
34. Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003. 14:S241–S245.

35. Roth-Eichhorn S, Heitmann B, Flemming P, Kubicka S, Trautwein C. Evidence for the decreased expression of the latent TGF-beta binding protein and its splice form in human liver tumors. Scand J Gastroenterol. 2001. 36:1204–1210.
36. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999. 13:9–22.

37. Kobayashi T, Liu X, Wen FQ, Fang Q, Abe S, Wang XQ, Hashimoto M, Shen L, Kawasaki S, Kim HJ, Kohyama T, Rennard Si. Smad3 mediates TGF-beta1 induction of VEGF production in lung fibroblasts. Biochem Biophys Res Commun. 2005. 327:393–398.
38. Yamaguchi K, Nishimura Y, Shigematsu S, Takeuchi Y, Nakamura J, Aizawa T, Hashizume K. Vascular endothelial cell growth factor attenuates actions of transforming growth factor-beta in human endothelial cells. J Biol Chem. 2004. 279:55104–55108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download