Abstract
Ionizing radiation produces reactive oxygen species, which exert diverse biological effects on cells and animals. We investigated alterations of heme oxygenase (HO) and non-protein thiols (NPSH), which are known as two major anti-oxidant enzymes, in female and male C57BL/6 mice in the lung, liver, and brain after whole-body γ-irradiation with 10 Gy (1-7 days) as well as in the lung after whole-thorax γ-irradiation (WTI) with 12.5 Gy (1-26 weeks). Most significant alteration of HO activity was observed in the liver, which elevated 250% in males. NPSH level in female liver was increased on the 5th-7th days but decreased in males on the 3rd day. In the lung, the elevation of HO activity in both sexes and the pattern of NPSH change were similar to that of the liver. On the other hand, the increase of HO activity on the 16th week and the decrease of NPSH level on the 2nd week were observed only in male lung after WTI. This study shows that the liver is the most sensitive tissue to γ-irradiation-induced alterations of HO activity in both female and male mice. In addition, there exists significant differential effect of γ-irradiation on anti-oxidant system in female and male mice.
Radiation has been used increasingly from discovery of x-ray over one hundred years ago in medicine, both in diagnosis and treatment. Clinically, radiation is applied to whole body for bone marrow transplantation and to limited area of the body for the cure of local cancer. Radiation produces reactive oxygen species (ROS), which can lead to the damage of lipids, proteins, carbohydrates and nucleic acids, and destroy cancer cells in the treated area. However, normal cells in the surrounded area can also be injured by radiation itself or even in the other site by circulating ROS.
Intracellular ROS are tightly controlled by different anti-oxidant defense systems. It has been demonstrated during the last decade that one of the important components of the anti-oxidant defense is heme oxygenase (HO) (1, 2). HO-1 (HO, EC 1.14.99.3) is identified as the 32-kDa stress (heat shock) protein (HSP32) (3, 4), that is a microsomal enzyme to catalyze oxidative breakdown of heme molecule to carbon monoxide (CO), Fe2+, and biliverdin, which is subsequently reduced to bilirubin by biliverdin reductase (5, 6). Cytoprotective role of HO-1 can be explained by the removal of free heme (a pro-oxidant) and the production of bilirubin, which is considered as a potent anti-oxidant (1, 2, 7, 8). Carbon monoxide is thought to be an active participant in signal transduction and possesses potent anti-inflammatory properties (9). Ferrous iron liberated in this reaction has pro-oxidant activity like free heme, however, intracellular chelatable iron in cytoplasm is sequestered by ferritin (10), preventing its participation in the oxidative processes.
The other key component involved in redox regulation of signal transduction and expression of anti-inflammatory anti-oxidant defense enzymes is reduced glutathione (γ-glutamylcysteinylglycine, GSH) (11-13). Beside participation in signal transduction, GSH plays extremely potent role in anti-oxidant defense because it possesses not only the direct radical-scavenging ability but also is the essential component of glutathione peroxidase system, which eliminates different hydroperoxides without free radical production (12). In animal tissues, GSH is the most abundant non-protein thiol (NPSH), comprising approximately 75-90% of NPSH (13-15). Other widespread NPSH are cysteine and coenzyme A.
Radiation therapy (RT) is a common modality used for the treatment of thoracic malignancies, but impaired healing of wounds in irradiated tissues or injury to surrounding normal lung can present clinical complications such as pneumonitis and accompanying fibrosis, occurring in up to 20% of patients irradiated for lung cancer, breast cancer, lymphoma, or thymoma (16). The aim of this study was therefore to investigate and compare the radiation-induced alterations of the important antioxidant enzymes such as HO and NPSH after whole-body γ-irradiation by lethal dose and to determine if any differences due to gender exist. In addition, especially long-term observation of the antioxidant levels in lung after local thorax γ-irradiation can give the information of radiation-induced side effects related with the alterations of these antioxidants.
Female and male C57BL/6 mice were purchased from Daehan Biolink (Korea) and 7-8 weeks old at the beginning of experiments. The mice were housed in conventional animal house conditions with 12-hr light-dark cycle, temperature of 22±2℃, relative humidity of 50%, standard pelleted diet and tap water ad libitum. The animal care, handling, and experimental procedures were conducted in accordance with the guidelines approved by the Animal Care and Use Committee of the Korea Institute of Radiological & Medical Sciences (KIRAMS).
Whole-body irradiation and local whole-thorax irradiation of the mice were performed using 60Co γ-rays from a Theratron 780 irradiation unit (Atomic Energy of Canada, Ltd., Canada). For whole-body irradiation, mice were placed into Plexiglas box and irradiated with a single dose of 10 Gy (0.5 Gy/min). For local whole-thorax irradiation, mice were anesthetized by i.p. injection of a mixture of ketamine (70 mg/kg) and xylazine (6 mg/kg), and fixed in a supine position on a Plexiglas tray. The thoracic regions (1.5 cm in length in cephalic-tail direction) of mice were placed in the field defined by collimators and irradiated with a single dose of 12.5 Gy (0.76 Gy/min). Other parts of the bodies were additionally shielded with 5-cm lead blocks. Before tissue sampling, irradiated and control mice were sacrificed by cervical dislocation in 1, 3, 5, and 7 days after whole-body irradiation and in 1, 2, 4, 12, 16, and 26 weeks after whole-thorax irradiation.
Immediately after sacrifice of mice by cervical dislocation, the cranial, peritoneal, and chest cavities of mice were opened, and the tissues (lung, liver, and brain) were quickly harvested and washed (except for brain) in cold 100 mM K-phosphate buffer (pH 7.4) containing 2 mM MgCl2 (MgCl2-phosphate buffer). The tissues were slightly dried by paper filter, minced with scissors, and homogenized in 4 volumes of MgCl2-phosphate buffer on ice by a Tissue-Tearor with a 7-mm probe (BioSpec Products, Inc., WI, U.S.A.). The homogenates were centrifuged at 1,500×g for 10 min at 4℃, and the obtained supernatants were further centrifuged at 15,000×g for 15 min at 4℃. The 15,000×g supernatants were kept at 4℃ and used for determination of HO activity not later than 1-1.5 hr after preparation. Protein concentration of the 15,000×g supernatants was determined by the method of Schacterle and Pollack (17) with bovine serum albumin as a standard. The reaction mixture for determination of HO activity included MgCl2-phosphate buffer, 412 mg of protein/mL of the 15,000×g supernatant, 1 mg of protein/mL of liver 105,000×g supernatant as a source of biliverdin reductase, 2 mM glucose 6-phosphate, 1 U glucose-6-phosphate dehydrogenase, 25 µM hemin, and 1 mM NADPH in a final volume of 500 µL in 1.7-mL Eppendorf microcentrifuge tubes. Reaction was initiated by the addition of NADPH, and the reaction mixtures were incubated in a water bath at 37℃ in the dark for 10 min (liver), 20 min (brain), or 40 min (lung). To terminate the reaction and extract produced bilirubin, 500 µL of chloroform were added to each sample, the samples were vortexed thoroughly for 30 sec, and centrifuged at 15,000×g for 10 min. The lower chloroform layer was transferred to a spectrophotometer microcuvette to measure the difference in optical density between 464 and 530 nm (ΔOD464-530) using UV/Visible spectrophotometer Ultrospec 3100 pro (Biochrom, Ltd., England). The extinction coefficient (ε464-530) of 40 mM/cm was used for calculation of bilirubin concentrations (18). HO activity was expressed in picomoles of bilirubin produced per milligram of protein per hour.
As a source of biliverdin reductase, 105,000×g supernatant of liver homogenate was used (18). To prepare the supernatant, livers of overnight-fasted non-irradiated mice were perfused in situ with cold (4℃) 1.15% KCl, harvested, slightly dried by paper filter, minced with scissors, and homogenized in 4 volumes of MgCl2-phosphate buffer on ice. The homogenates were centrifuged at 1,500×g for 10 min at 4℃, and the pooled supernatant was further centrifuged at 15,000×g for 15 min at 4℃ and then at 105,000×g for 1.5 hr at 4℃. The 105,000×g supernatant was diluted to 5 mg of protein/mL with MgCl2-phosphate buffer and stored at -20℃.
Semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) was used for the mRNA expression level determination. After thawing liver samples frozen in liquid nitrogen, RNA was purified from the liver using RNAzol (Tel-Test, Friendswood, U.S.A.), and RNA concentration was spectrophotometrically determined by absorbance at 260 nm. One microgram of intact RNA was reverse-transcribed into cDNA in the reaction mixture containing 50 mM Tris-HCl (pH 8.3), 3 mM MgCl2, 75 mM KCl, 2.5 µg/mL pd(N)6 primer, 0.5 mM each of dNTP, and 10 U of AMV-RT (Amersham). Then produced cDNA was amplified by polymerase chain reaction (PCR) in the mixture containing 10 µL cDNA template from RT reaction, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5mM MgCl2, 0.5mM each of dNTP, 1.0 µM of each primer, and 0.5 U Tag DNA polymerase (Takara, Tokyo, Japan). The oligonucleotide primers used in these experiments are as followings; HO-1 PCR primer (5'-primer 5'-AACAAGCAGAACCCAGTC-3', 3'-primer: 5'-TGTCATCTCCAGAGT-GTTC-3'), β-actin primer (5'-primer: 5'-TGGAATCCTGTGGCATCCATGAAA-3', 3'-primer: 5'-TAAAACGCAGCTCAGTAACAGTCCG-3'). PCR was performed with a DNA thermal cycle (Hybaid, Ashford, U.K.) at 94℃ for 30 sec, at 56℃ for 30 sec, and at 72℃ for 30 sec per cycle. The PCR products were visualized by electrophoresis on 1% agarose gel in the presence of 0.5 µg/mL ethidium bromide. Density of bands was measured using an image analyzer (Flour-S Multimager, Bio-Rad, CA, U.S.A.).
NPSH content in animal tissues was determined with DTNB by the method of Sedlak and Lindsay (19). Briefly, immediately after sacrifice of mice, the tissues (50-90 mg of liver, 100-150 mg of brain and lung) were quickly harvested, placed on ice, slightly dried by paper filter (except for brain), weighed, minced with scissors, and homogenized on ice in a mixture of 2 mL of 10% TCA and 2 mL of 0.02 M EDTANa2. The homogenates were centrifuged at 3,500×g for 20 min at 4℃. Then, 0.5 mL of the supernatant was mixed with 1 mL of 0.4 M Tris-HCl buffer (pH 8.9) containing 0.02 M EDTANa2, and 25 µL of 0.01 M DTNB in methanol were added to each sample. OD was measured at 412 nm in 5 min. Blank sample contained all the above-mentioned components, except animal tissue. NPSH contents were calculated using an extinction coefficient (ε412) of 13.1 mM/cm and expressed in micromoles per gram of wet tissue weight.
All experimental data were evaluated by GraphPad Prism software (San Diego, CA, U.S.A.). In the most of experiments, the significances of differences between irradiated and control groups were tested using Student's t test or Mann-Whitney when the data failed to pass a normality test. The effect of irradiation on HO-1 mRNA level was analyzed by Kruskal-Wallis test. Differences among the groups were considered as statistically significant at p<0.05.
Comparison of HO activity and NPSH level in lung, liver and brain of male and female control mice demonstrated that HO activity was the highest in brain and the lowest in lung; NPSH level was approximately same in lung and brain, and much higher in liver (Fig. 1). The significant difference between sexes was observed only in hepatic NPSH content: it was approximately 15% higher in males than in females.
In brain, HO activity in males was slightly decreased on the 5th day but no alteration of that in females (Fig. 2). Significant elevation of HO activity (approximately 25-30%) in lung was observed on the 5th day in females and on the 7th day in males after irradiation. The most impressed radiation-induced alterations of HO activity occurred in livers of both male and female mice. HO activity began to increase on the 3rd day after the irradiation in females and on the 5th day in males, and reached nearly 200% of the control level in females and 250% in males on the 7th day. To further confirm whether the elevation of HO activity is accompanied with the increase of gene expression of HO-1, we performed the RT-PCR for HO-1 mRNA in liver. As shown in Fig. 3, mRNA expression of HO-1 was significantly elevated on the 5th day and persisted until 7 days after irradiation (8.7 and 8.0-fold compared to the control group respectively).
NPSH level in the brain was not significantly changed in both male and female mice (Fig. 4). In case of the lung, NPSH level was significantly decreased on the 1st day after irradiation in males but increased in females. Like, irradiation induced different response of NPSH level in livers of males and females; it was increased in females on the 5-7th days in sharp contrast to decrease in males on the 3rd day. The alteration time of NPSH level in livers was shown later than that in lungs even the pattern of NPSH response was similar.
HO activity and NPSH level in the lung after whole-thorax irradiation at a dose of 12.5 Gy were evaluated during 26 weeks after exposure. As shown in Fig. 5, the whole-thorax irradiation resulted in different reaction of males and females. In females, there were no significant changes of both HO activity and NPSH level, although HO activity showed a tendency to increase on the 1st week and to decrease on the 12th week after irradiation. However, NPSH level was significantly decreased on the 2nd week and HO activity was increased on the 16th week (by 45%) after irradiation in male mice.
Cellular and tissue resistance against ionizing radiation depends on many endogenous parameters, including anti-oxidant systems, and their capacity for adaptive response. One of such defense systems is HO. There are numerous studies on high response of HO to various oxidant stimuli in different animal tissues (1, 2, 6, 9), however, only a few reports about HO response to ionizing radiation. Two-fold elevated HO activity was demonstrated in rat liver on the 5-7th days after 7 Gy whole-body X-irradiation (20). The transient dose-dependent 2 to 5.5 fold increase of HO-1 mRNA expression, HO-1 protein level and HO activity was shown in rat liver within a few hours after whole-body X-irradiation with doses varying from 4 to 21.7 Gy (21). Bilateral renal X-irradiation with 20 Gy dose increased HO-1 protein expression 6-10 fold in rat glomeruli on the 50th and 65th days (22). Gammairradiation with 0.1-10 Gy caused 2-4 fold increase of HO-1 mRNA expression in human skin fibroblasts in vitro (23).
In the present study, we investigated HO activity in the lung, liver and brain after whole-body irradiation. Brain exclusively possesses HO-2 which can not be induced by oxidative stimuli (9), whereas the lung and liver have a large fraction of stress-inducible HO-1 in their total HO pool (5, 24). It was interest to compare the radiation effects on these different organs from the light of HO activity, one of the main antioxidant systems. Furthermore, additional long-term investigations of HO activity in the lung after whole-thorax irradiation with a single dose of 12.5 Gy were also performed. The reason of employing 12.5 Gy for whole-thorax irradiation was that it has often been used to induce the pulmonary injury in fibrosis-sensitive C57BL/6 mice (25, 26).
Our data showed that whole-body γ-irradiation with 10 Gy dose caused the significant increment of HO activity in the lung of both female and male mice. This increase of HO activity was preceded by the decrease of NPSH level on the 1st day after irradiation in males. It is known that the depletion of the main NPSH, GSH, can promote the expression of HO-1 either through the intensified accumulation of ROS or the direct influence of GSH or GSSG on signal transduction pathways (11, 25). In our experiments, such interrelationship between NPSH level and HO activity was observed only in males, but not in females.
After whole-thorax irradiation with 12.5 Gy, HO activity in the lung of male mice was elevated almost 1.5-fold on the 16th week and NPSH level was significantly decreased as early time as 2nd week. However, no significant alterations of HO activity as well as NPSH level were shown in any time points in females. The appearance of increased HO activity in the irradiated lung of male mice was well accordance with other results (26, 27), supporting the view point of that HO-1 may have a universal protective role in inflammation. The presence of a low concentration of CO, a marker of HO activity, may protect against bleomycin-induced lung injury in mice (28). Moreover, it is interesting to note that men are significantly less likely to develop severe radiation pneumonopathy than women who received chemoradiation (29).
The radiation-induced elevation of HO activity, HO-1 mRNA or protein expression in the liver after whole-body irradiation was previously reported in rats (20, 21). Here we found that the marked increase of HO activity in the liver occurred in several days after whole-body irradiation with lethal dose in both male and female mice, being slightly more expressed in males than in females. We suggest that this effect might be connected with stronger exposure of the liver to heme which was derived from damaged erythrocytes or destabilized membrane-bound tissue hemoproteins than other organs. Powerful pro-oxidant free heme causes a sharp burst of oxidative processes, particularly lipoperoxidation, and is known as a potent inducer of HO-1 (1). Earlier it was shown that the increase of the hepatic free heme level preceded the elevation of HO activity in the liver of X-irradiated rats (20). The increase of HO activity in the liver was accompanied with significantly elevated the level of HO-1 mRNA, suggesting the presence of the second wave for the upregulation of HO-1 gene (on the 5-7th days) in addition to the first wave in early period (during several hours) after irradiation that was observed by other investigators (21). Unlike HO activity, the changes of NPSH level in the liver of male and female mice were markedly different; in males the increase of HO activity was preceded by the decrease of NPSH level, while there was no decrease of NPSH level in females and the increase of HO activity and NPSH level was occurred together.
In the brain, no increase of HO activity after whole-body irradiation was observed in both male and female mice, and there was even rather slight decrease in males on the 5th day. It is known that the brain has higher basal level of HO activity than the liver or lung (9) (Fig. 1A), and also essentially different composition of HO pool; in normal conditions HO-1 is almost absent and HO-2 is the dominant form of HO in the brain (9), whereas in the liver and lung HO-1 constitutes considerable part of total HO pool (5, 24). Unlike HO-1, HO-2 is not inducible by oxidative stress but may be induced by adrenal glucocorticoids (9). The high level of HO activity due to the large amount of HO-2 in the brain and the absence of HO-1 could interpret the resistance of the brain against radiation and negligible alterations of HO activity. The slight decrease of HO activity observed in the brain might have been caused by perturbation of hormonal status after irradiation.
In summary, to our knowledge, this is the first study to compare the radiation-induced effects on HO activity in different tissues of female and male mice. Among the investigated tissues, the most pronounced changes of HO activity after lethal whole-body γ-irradiation were found in the liver, whereas the brain was the most resistant in this respect. After whole-thorax γ-irradiation with fibrosis-inducing dose, the significant increase of HO activity was observed only in males. Probable mechanisms of the radiation-induced alterations of HO system in the different tissues as well as sexual differences are investigated in further study.
Figures and Tables
Fig. 1
HO activity (A) and NPSH level (B) in the lung, liver, and brain of control female and male C57BL/6 mice. Sixteen mice were used in each group. All data are shown as means±SEM. *p<0.05 compared to female mice.

Fig. 2
HO activity in the brain, lung and liver of female (A) and male (B) C57BL/6 mice after whole-body irradiation with 10 Gy. Each time point test group was compared with own control group. Four mice were used in each test and corresponding control group. All data are shown as means±SEM. *p<0.05, †p<0.01, ‡p<0.001 compared to corresponding control group.

Fig. 3
HO-1 mRNA expression levels in the liver of female C57BL/6 mice after whole-body irradiation with 10 Gy. (A) The purified total RNA was converted to cDNA and amplified by reverse transcriptase polymerase chain reaction (RT-PCR). The amplified RT-PCR product was visualized on 1% agarose gel. (B) The band density was quantified in comparison to the β-actin mRNA expression using image analyzer. Four mice were used in each test and control group. All data are shown as means±SEM. *p<0.05, †p<0.01 compared to control group.
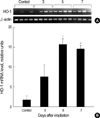
Fig. 4
NPSH contents in the brain, lung and liver of female (A) and male (B) C57BL/6 mice after whole-body irradiation with 10 Gy. Each time point test group was compared with own control group. Four mice were used in each test and corresponding control group. All data are shown as means±SEM. *p<0.05, †p<0.01 compared to corresponding control group.

Fig. 5
HO activity (A, B) and NPSH content (C, D) in the lung of female (A, C) and male (B, D) C57BL/6 mice after whole-thorax irradiation with 12.5 Gy. Each time point test group was compared with own control group. Four mice were used in each test and corresponding control group. All data are shown as means±SEM. *p<0.05 compared to corresponding control group.

ACKNOWLEDGEMENTS
We are grateful to Mr. Sin-Keun Kang, Mr. In-Sung Jung, and Ms. Ji-Young Shim for technical assistance.
References
1. Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000. 28:289–309.
2. Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L1029–L1037.

3. Taketani S, Kohno H, Yoshinaga T, Tokunaga R. The human 32-kDa stress protein induced by exposure to arsenite and cadmium ions is heme oxygenase. FEBS Lett. 1989. 245:173–176.

4. Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989. 86:99–103.

5. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988. 2:2557–2568.

6. Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996. 6:129–168.

7. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987. 235:1043–1046.

8. Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000. 348:615–619.

9. Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997. 37:517–554.
10. Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA. 1994. 91:2607–2610.

11. Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med. 2000. 28:1405–1420.

12. Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002. 64:1019–1026.

13. Moran LK, Gutteridge JM, Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem. 2001. 8:763–772.

14. Sedlak J, Hanus L. Changes of glutathione and protein bound SH-groups concentration in rat adrenals under acute and repeated stress. Endocrinol Exp. 1982. 16:103–109.
15. Sedlak J. Long-term effect of hypophysectomy on various fractions of sulfhydryl groups in thyroid, adrenal and some other organs in rats. Endocrinol Exp. 1985. 19:186–192.
16. Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 2000. 10:296–307.

17. Schacterle GR, Pollack RL. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973. 51:654–655.

18. Maines MD, Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978. 253:2321–2326.

19. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968. 25:192–205.

20. Deev LI, Topchishvili GI, Akhalaia MI, Platonov AG. Effect of X-ray irradiation on the activity of key enzymes in heme biosynthesis and breakdown in the rat liver. Biull Eksp Biol Med. 1985. 99:681–683.
21. Suzuki K, Mori M, Kugawa F, Ishihara H. Whole-body X-irradiation induces acute and transient expression of heme oxygenase-1 in rat liver. J Radiat Res (Tokyo). 2002. 43:205–210.

22. Datta PK, Moulder JE, Fish BL, Cohen EP, Lianos EA. Induction of heme oxygenase 1 in radiation nephropathy: role of angiotensin II. Radiat Res. 2001. 155:734–739.

23. Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991. 51:974–978.
24. Dennery PA, Rodgers PA, Lum MA, Jennings BC, Shokoohi V. Hyperoxic regulation of lung heme oxygenase in neonatal rats. Pediatr Res. 1996. 40:815–821.

25. Johnston CJ, Wright TW, Rubin P, Finkelstein JN. Alterations in the expression of chemokine mRNA levels in fibrosis-resistant and -sensitive mice after thoracic irradiation. Exp Lung Res. 1998. 24:321–337.

25. Lautier D, Luscher P, Tyrrell RM. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992. 13:227–232.

26. Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, Willich N, Rube C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000. 47:1033–1042.
26. Willis D, Moore AR, Willoughby DA. Heme oxygenase isoform expression in cellular and antibody-mediated models of acute inflammation in the rat. J Pathol. 2000. 190:627–634.

27. Rizzardini M, Terao M, Falciani F, Cantoni L. Cytokine induction of haem oxygenase mRNA in mouse liver. Interleukin 1 transcriptionally activates the haem oxygenase gene. Biochem J. 1993. 290:343–347.

28. Morse D. The role of heme oxygenase-1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003. 29:Supple 3. S82–S86.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download