Abstract
Osteoclast-like giant cell tumor of the pancreas is a very rare neoplasm, of which the histiogenesis remains controversial. A 63-yr-old woman was hospitalized for evaluation of epigastric pain. An abdominal computerized tomography revealed the presence of a large cystic mass, arising from the tail of pancreas. A distal pancreatectomy with splenectomy was performed. Histologically, the tumor was composed of mononuclear stromal cells intermingled with osteclast-like giant cells. In addition, there was a small area of moderately to well differentiated ductal adenocarcinoma. The final pathologic diagnosis was osteoclast-like giant cell tumor of the pancreas with ductal adenocarcinoma. Here, we describe the histopathological, immunohistochemical, ultrastructural and molecular biological findings of this tumor with review of the literature pertaining to this condition.
Giant cell tumor of the pancreas is a very rare neoplasm and appears as two different phenotypes: undifferentiated carcinoma and osteoclast-like giant cell tumor (OGTP) (1-3). OGTP, mimicking giant cell tumor of bone should be distinguished from undifferentiated carcinoma. In contrast to OGTP, undifferentiated carcinoma exhibit more pleomorphic and anaplastic features. OGTP is the more rare type and has a better prognosis than undifferentiated carcinoma (1-3). An implication of these differences in histological and clinical characteristics is that these tumors have a different histogenesis. To date, generally, undifferentiated carcinoma is believed to have an epithelial origin without debating. However, the histogenesis of OGTP remains controversial either epithelial or mesenchymal derivation in the literature (4-16).
Here, we report a case of OGTP, performed the histopathological, immunohistochemical, ultrastructural, and molecular biological studies and review the literature pertaining to this condition.
A 63-yr-old woman was admitted to Chonnam National University Hospital with a three month history of epigastric pain. Physical examination showed a smooth palpable mass in the epigastrium. The laboratory examinations were within normal range. An abdominal computerized tomography revealed the presence of a large cystic mass, arising from the tail of pancreas (Fig. 1A). A distal pancreatectomy with splenectomy was performed. The resected tumor was a 8×7×6-cm hemorrhagic and cystic mass in the tail of the pancreas, mimicking pancreatic pseudocyst (Fig. 1B). The histology confirmed OGTP with ductal adenocarcinoma. She received third courses of chemotherapy with gemcitabine. She has been well, without evidence of tumor recurrence 6 months after surgery.
Histologically, the tumor was composed of typical ductal adenocarcinoma and surrounding mononuclear stromal cells intermingled with osteoclast-like giant cells and pleomorphic large cells (Fig. 2). The mononuclear cells demonstrated irregular, pleomorphic nuclei with varying degrees of cytologic atypia. In contrast, osteoclast-like giant cells had multiple small uniform nuclei without atypia. Only some pleomorphic large cells showed atypical mitoses. In addition, there was a small area of moderately to well differentiated ductal adenocarcinoma. Frequently cystic degeneration was found in this tumor.
All procedures for immunohistochemical staining were done by the Micro-Probe staining system (Fisher Scientific, Pittsburgh, PA, U.S.A.) based on capillary action (17). Paraffin sections, of 4 µm in thickness with mounted probe on slides, were immunostained with the panel of primary antibodies listed Table 1, by the avidin-biotin peroxidase complex method. The results of immunohistochemical stainings are summarized in Table 2. Immunohistochemically, cytokeratin (CK) and epithelial membrane antigen (EMA) are strongly positive for ductal carcinoma cells, whereas both osteoclast-like giant cells and mononuclear cells were negative for CK (Fig. 3A) and EMA. Osteoclast-like giant cells and mononuclear cells were positive for histiocytic markers, CD-68 (Fig. 3B) and lysozyme. In general, immunoreactivity of vimentin was more intense in pleomorphic large cells than that of osteoclast-like giant cells and mononuclear cells. Most of the nuclei in ductal carcinoma cells expressed PCNA, demonstrating intense proliferative activity, while no nuclei were stained in osteoclast-like giant cells and mononuclear cells. Anti-p53 antibody did react with some atypical mononuclear cells of weak intranuclear staining, but did not react with either ductal carcinoma cells or osteoclast-like giant cells.
For electron microscopy, the tissue specimen was fixed in 2.5% glutaraldehyde, fixed in osmium tetroxide, and embedded in epon mixture. Ultrathin sections, 80 nm in thickness, were made by LKB-V ultramicrotome with diamond knife, stained with uranyl acetate and lead citrate, and examined with a JEM 100CX type II electron microscope. The nucleus of the mononuclear cell contained prominent nucleoli, with dispersed clumps of heterochromatin. The cytoplasm contained ample mitochondria, and moderate amount of rough endoplasmic reticulum (Fig. 4) (Table 2). But we did not find the evidence of epithelial differentiation including microvilli and desmosomes. In the ultrastructure of multinucleated osteoclast-like giant cell, there were multiple nuclei with dispersed chromatin and a fine chromatin rim. The cytoplasm was characterized by abundant mitochondria of varying size, free ribosomes, and dilated empty rough endoplasmic reticulum cisternae (Fig. 5) (Table 2).
To analyze K-ras gene mutations, we microdissected in both ductal carcinoma and giant cell tumor components separately. After extracting DNA, polymerase chain reaction (PCR) amplification and direct sequencing were done to detect K-ras gene mutation using ABI 3700DNA analyzer. The size of amplified PCR product was 223 bp. All mutations were verified in both the sense and anti-sense directions. DNA from ductal carcinoma with the known K-ras mutation was used as a positive control. A microdissection method was applied to allow analyses of K-ras gene mutations as performed by Sakai et al. (15). However, none of the microdissected osteoclast-like giant cell, mononuclear cell, and ductal carcinoma cell contained K-ras gene mutations at codon 12 (GGT) and 13 (AGT) in our case (Table 2).
OGTP is very rare and comprises less than 1% of nonendocrine pancreatic tumor (1-3). To date, only two cases have been reported in Korea (18, 19). In the review of English literature (20-22), OGTP tends to present in the 7th decade of life, although younger patients have been reported. There was no sex preference. The main symptoms and signs were abdominal pain, palpable mass, weight loss and jaundice. OGTP commonly exhibited as large cystic neoplasms with hemorrhage and necrosis of variable extent. There was predilection to involve head and body portion of the pancreas. The prognosis of OGTP is better than that of pancreatic ductal adenocarcinoma and undifferentiated carcinoma, suggesting that OGTP grows more slowly and is less likely to metastasize than pancreatic ductal adenocarcinoma and undifferentiated carcinoma. However, the outcome of OGTP is extremely variable in the literature. Although it is difficult to decide treatment modality because of its rarity, the choice of treatment of OGTP may be surgical resection if possible. Also, in previous reports, experience of adjuvant treatments including chemotherapy or radiotherapy was not described, so no detailed information was provided. However, because the outcome of OGTP is extremely variable, it may be reasonable to consider adjuvant treatment for prevention of tumor recurrence. In our case, we did attempt chemotherapy with gemcitabine, according to treatment protocol established for pancreatic ductal adenocarcinoma. We think that this attempt is important and necessary to detail the treatment modalities and clinical outcome.
Although the histiogenesis of OGTP has been previously investigated by immunohistochemical, ultrastructural, and molecular biologic studies, the origin of these cells is still controversial.
In our case, osteoclast-like giant cells and mononuclear cells were positive for histiocytic markers, CD68 and lysozyme in contrast to negative for epithelial markers, CK and EMA. In the ultrastructure of multinucleated, osteoclast-like giant cells and mononuclear cells, we did not find either microvilli or desmosomes for the clue of epithelial differentiation.
Tumor progression is regulated by many biological processes including cell proliferation and genetic alterations of tumor suppressor gene and oncogene. PCNA reflects cell proliferation and mutation of the p53 tumor suppressor gene represents one of the common genetic alterations in pancreatic ductal carcinoma. In our case, ductal carcinoma cells expressed PCNA while no nuclei were stained in osteoclast-like giant cells and mononuclear cells. p53 immunoreactivity showed only weak intranuclear staining in some atypical mononuclear cells, but did not show in ductal carcinoma cells and osteoclast-like giant cells.
K-ras gene mutations are a hallmark of pancreatic ductal carcinoma and appear to be present in 80% to 90% of tumors. Evidence of K-ras gene mutations indicates that its origin is an epithelial nature. But in our case, none of the microdissected osteoclast-like giant cell, mononuclear cell, and ductal carcinoma cell contained K-ras gene mutations.
These results may support that both osteoclast-like giant cells and mononuclear cells in OGTP originate from mesenchymal rather than epithelial cell. However, although most reports have debated the histiogenesis of OGTP from immunohistochemical, ultrastructural, and molecular biologic aspects, it is still obscure whether the histiogenesis of OGTP is epithelial or mesenchymal origin (4-16). There are several possible explanations for these discrepancies. First, OGTP is too rare to discern differences in the origin of these cells. Second, immunohistochemical staining methods and primary antibodies used are different in different cases (4). Third, OGTP can express different phenotypes with variable stages and degrees of differentiation during tumor progression (4, 23). Fourth, circulating precursor cell may be stimulated by tumor derived factors such as cytokines, to proliferate and differentiate into different cell types (4, 16, 20, 21).
Clinical characteristics and histiogenesis of OGTP are still now variable and controversial in the literature. Therefore, further studies based on a large populations are necessary to clarify its histiogenesis.
Figures and Tables
Fig. 1
(A) An abdominal computerized tomography reveals the presence of a large cystic mass, arising from the tail of pancreas. (B) The resected tumor is a 8×7×6-cm hemorrhagic and cystic mass in the tail of the pancreas.
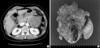
Fig. 2
Histologically, the tumor is composed of typical ductal adenocarcinoma (closed arrow) and surrounding mononuclear stromal cells (open arrow) intermingled with osteoclast-like giant cells (closed arrow head) and pleomorphic large cells (open arrow head) (H&E ×200).

Fig. 3
Immunohistochemical staining of cytokeratin (A) and CD-68 (B). Cytokeratin (CK) is strongly positive for ductal carcinoma cells, whereas both osteoclast-like giant cells and mononuclear cells are negative for CK. However, osteoclast-like giant cells and mononuclear cells are positive for histiocytic markers, CD-68 (×200).

Fig. 4
Electron microscopically, the nucleus of the mononuclear cell contains prominent nucleoli, with dispersed clumps of heterochromatin. The cytoplasm contained ample mitochondria, and moderate amount of rough endoplasmic reticulum (×4,000).

Fig. 5
Electron microscopically, osteoclast-like giant cell has multiple nuclei with dispersed chromatin and a fine chromatin rim. The cytoplasm is characterized by abundant mitochondria of varying size, free ribosomes, and dilated empty rough endoplasmic reticulum cisternae (×4,000).

References
1. Rosai J. Carcinoma of pancreas simulating giant cell tumor of bone: electron-microscopic evidence of its acinar cell origin. Cancer. 1968. 22:333–344.

2. Alguacil-Garcia A, Weiland LH. The histologic spectrum, prognosis, and histogenesis of the sarcomatoid carcinoma of the pancreas. Cancer. 1977. 39:1181–1189.

3. Lewandrowski KB, Weston L, Dickersin GR, Rattner DW, Compton CC. Giant cell tumor of the pancreas of mixed osteoclastic and pleomorphic cell type: evidence for a histogenetic relationship and mesenchymal differentiation. Hum Pathol. 1990. 21:1184–1187.

4. Sun AP, Ohtsuki Y, Liang SB, Sonobe H, Iwata J, Furihata M, Takeuchi T, Qiu Y, Chen BK, Watanabe R, Ohmori K. Osteoclast-like giant cell tumor of the pancreas with metastases to gallbladder and lymph nodes. A case report. Pathol Res Pract. 1998. 194:587–594.

5. Machado MA, Herman P, Montagnini AL, Jukemura J, Leite KR, Machado MC. Benign variant of osteoclast-type giant cell tumor of the pancreas: importance of the lack of epithelial differentiation. Pancreas. 2001. 22:105–107.

6. Hansen T, Burg J, Kirkpatrick CJ, Kriegsmann J. Osteoclast-like giant cell tumor of the pancreas with ductal adenocarcinoma: case report with novel data on histogenesis. Pancreas. 2002. 25:317–320.

7. Berendt RC, Shnitka TK, Wiens E, Manickavel V, Jewell LD. The osteoclast-type giant cell tumor of the pancreas. Arch Pathol Lab Med. 1987. 111:43–48.
8. Dizon MA, Multhaupt HA, Paskin DL, Warhol MJ. Osteoclastic giant cell tumor of the pancreas: an immunohistochemical study. Arch Pathol Lab Med. 1996. 120:306–309.
9. Dworak O, Wittekind C, Koerfgen HP, Gall FP. Osteoclastic giant cell tumor of the pancreas. An immunohistological study and review of the literature. Pathol Res Pract. 1993. 189:228–231.
10. Watanabe M, Miura H, Inoue H, Uzuki M, Noda Y, Fujita N, Yamazaki T, Sawai T. Mixed osteoclastic/pleomorphic-type giant cell tumor of the pancreas with ductal adenocarcinoma: histochemical and immunohistochemical study with review of the literature. Pancreas. 1997. 15:201–208.
11. Gatteschi B, Saccomanno S, Bartoli FG, Salvi S, Liu G, Pugliese V. Mixed pleomorphic-osteoclast-like tumor of the pancreas. Light microscopical, immunohistochemical, and molecular biological studies. Int J Pancreatol. 1995. 18:169–175.
12. Imai Y, Morishita S, Ikeda Y, Toyoda M, Ashizawa T, Yamamoto K, Inoue T, Ishikawa T. Immunohistochemical and molecular analysis of giant cell carcinoma of the pancreas: a report of three cases. Pancreas. 1999. 18:308–315.
13. Martin A, Texier P, Bahnini JM, Diebold J. An unusual epithelial pleomorphic giant cell tumour of the pancreas with osteoclast-type cells. J Clin Pathol. 1994. 47:372–374.

14. Westra WH, Sturm P, Drillenburg P, Choti MA, Klimstra DS, Albores-Saavedra J, Montag A, Offerhaus GJ, Hruban RH. K-ras oncogene mutations in osteoclast-like giant cell tumors of the pancreas and liver: genetic evidence to support origin from the duct epithelium. Am J Surg Pathol. 1998. 22:1247–1254.
15. Sakai Y, Kupelioglu AA, Yanagisawa A, Yamaguchi K, Hidaka E, Matsuya S, Ohbuchi T, Tada Y, Saisho H, Kato Y. Origin of giant cells in osteoclast-like giant cell tumors of the pancreas. Hum Pathol. 2000. 31:1223–1229.

16. Goldberg RD, Michelassi F, Montag AG. Osteoclast-like giant cell tumor of the pancreas: immunophenotypic similarity to giant cell tumor of bone. Hum Pathol. 1991. 22:618–622.

17. Reed JA, Manahan LJ, Park CS, Brigati DJ. Complete one-hour immunocytochemistry based on capillary action. Biotechniques. 1992. 13:434–443.
18. Sung SH, Han WS. Fine needle aspiration cytology of osteoclastic giant cell yumor of the pancreas. Korean J Cytopathol. 1998. 9:89–94.
19. Song HG, Kim YI, Yu ES, Lee HS. Two histologic variants of giant cell carcinoma of the pancreas. Korean J Pathol. 1987. 21:192–198.
20. Shiozawa M, Imada T, Ishiwa N, Rino Y, Hasuo K, Takanashi Y, Nakatani Y, Inayama Y. Osteoclast-like giant cell tumor of the pancreas. Int J Clin Oncol. 2002. 7:376–380.

21. Leighton CC, Shum DT. Osteoclastic giant cell tumor of the pancreas: case report and literature review. Am J Clin Oncol. 2001. 24:77–80.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download