Abstract
We developed age, gender and ethnic specific brain templates based on MR and Positron-Emission Tomography (PET) images of Korean normal volunteers. Seventy-eight normal right-handed volunteers (M/F=49/29) underwent 3D T1-weighted SPGR MR and F-18-FDG PET scans. For the generation of standard templates, an optimal target brain that has the average global hemispheric shape was selected for each gender. MR images were then spatially normalized by linear transformation to the target brains, and normalization parameters were reapplied to PET images. Subjects were subdivided into 2 groups for each gender: the young/midlife (<55 yr) and the elderly groups. Young and elderly MRI/PET templates were composed by averaging the spatially normalized images. Korean templates showed different shapes and sizes (mean length, width, and height of the brains were 16.5, 14.3 and 12.1 cm for man, and 15.6, 13.5 and 11.4 cm for woman) from the template based on Caucasian (18.3, 14.2, and 13.3 cm). MRI and PET templates developed in this study will provide the framework for more accurate stereotactic standardization and anatomical localization.
Advances in imaging technology in the past decades have allowed profound insights into the human brain function and anatomy for normal and pathological conditions. This has also led to advanced understanding of various neurological and neuropsychiatric diseases (1-7). Standard templates of brain images provide a standard framework in which the population-based assessment of brain function and anatomy is possible by incorporating inter-subject standardization (spatial normalization) of individual brain onto the standard template (8-12).
The most commonly used human brain templates for brain mapping studies are those of Talairach and Tournoux, which has Brodmanns' area labeled (13, 14). The Talairach template, however, does not properly represent the in vivo anatomy of subjects since it is based on post mortem sections of a 60-yr-old female subject. The variable slice separations, from 3 to 4 mm, and inconsistency in the data from the orthogonal planes are other limitations of the Talairach template for use as a standard template for brain mapping studies. The Montreal Neurological Institute (MNI) constructed a new standard brain to address the limitations of the Talairach template by averaging the large series of MRI scans on 305 young normal controls (239 males and 66 females, age 23.4±4.1 yr) whose scans were spatially normalized into the Talairach system by linear transformation (8, 11). The MNI templates have been used as a new international standard since they were adopted by the Statistical Parametric Mapping (SPM, Institute of Neurology, University College of London, U.K.) program, and these templates are the most widely used for human brain mapping studies (15, 16).
Although there have been remarkable advances in spatial normalization techniques, the differences in the shape of the hemispheres and the sulcal pattern of brains relative to age, gender, races, and diseases cannot be fully overcome by the nonlinear spatial normalization techniques (11). Spurious registration may be made more frequently for PET and SPECT images, the intensity of which are determined by both the functional and anatomical changes if the spatial normalization techniques that registered the images, based on intensity similarity, are used. The imperfect compensation for shape difference may lead to erroneous results in the anatomical localization, and this can lessen the accuracy and power of statistical analysis on the spatially normalized images.
Those limitations in the universal use of the currently available standard brain templates have led us to develop age, gender and ethnic specific anatomical and functional brain templates that are based on brain MR and PET images of Korean healthy normal volunteers.
Seventy-eight normal right-handed volunteers (M/F=49/29) that were without a lifetime history of any neurological, psychiatric or significant medical illnesses and past history of substance abuse were recruited from the local community through the Health Promotion Center at Seoul National University Hospital or from newspaper advertisements. All the participants were screened with the Korean version of modified Mini-Mental State Exam, Mood Evaluation Scale, and a simplified version of the handedness test. The subjects ranged in age from 18 to 77 yr (mean age=44.6±19.4 yr), and they were subdivided into 2 groups for each gender: the young/midlife group (<55 yr; 35 men, 13 women) and the elderly group (>55 yr; 14 men, 16 women). Although the selection of age 55 is arbitrary, this age has been used in the past as a threshold age to discriminate elderly subjects from the young/midlife subjects in many cognitive and imaging researches. There was no definite criterion for this age parameter.
Demographic information for each group, and the numbers of MRI and PET scans performed for the subjects in each group are shown in Table 1.
For all the volunteers, three-dimensional T1-weighted spoiled gradient echo (SPGR) MR images were acquired on a 1.5 Tesla GE SIGNA Scanner (GE Medical Systems, Milwaukee, IL, U.S.A.). Imaging parameters were as follows: 1.5 mm sagittal slices, echo time=5.5 msec, repetition time=14.4 msec, number of excitations=1, rotation angle=20 degrees, field of view=24×24 cm, and a matrix of 256×256.
Among 78 volunteers, 47 volunteers had F-18-FDG brain PET scans performed, using an ECAT EXACT 47 PET scanner (Siemens-CTI, Knoxville, TN, U.S.A.), with an intrinsic resolution of 5.2 mm FWHM (full width at half maximum). Images were simultaneously collected for 47 contiguous planes with a thickness of 3.4 mm, to give a 16.2 cm longitudinal field of view. Before FDG administration, transmission scanning was performed using three Ge-68 rod sources for attenuation correction. Static emission scans began 30 min after the injection of 370 MBq FDG and the scans continued for 30 min in the resting state (in a dark and quiet environment with the subject's eyes closed and their ears undisturbed by noise). Transaxial images were reconstructed using a filtered back-projection algorithm with a Shepp-Logan filter at a cutoff frequency of 0.3 cycles/pixel as 128×128×47 matrices of size 2.1×2.1×3.4 mm.
For the generation of standard templates, an optimal target brain that has the average global hemispheric shape was selected for each gender. To quantitatively evaluate the global hemispheric shape, intercommissural (anterior commissure-posterior commissure, AC-PC), verticofrontal, and mid-sagittal planes that are defined in the Talairach and Tournoux atlas and cortical peripheries were determined on every MR image, and the distances from these basal lines to the cortical peripheries were measured. Two different methods were used to determine the basal lines and cortical peripheries: manual and automatic methods. Fig. 1 is a flowchart for the strategy used in this study to select optimal target brains.
First, an image specialist determined the basal lines and cortical peripheries mannually using image registration software, FIRE (Functional Image REgistration, Seoul National University, Seoul, Korea), which allows for the interactive rigid body transformation of images with 6 degrees-of-freedom. In this program, 3 cross-reference views (axial, coronal, and sagittal) of the image are displayed, and the center of each view is set to be the origin of the image volume. Images can be rotated and translated by pushing the control buttons or drawing lines using the computer mouse. The magnitude of the rotation angle and translation distance can be varied from 0.1 to 270 degrees and mm, respectively. Each MR image was reoriented to the virtual x-y-z coordinates so that the AC-PC line of the brain was matched to the y-axis, the VAC line (vertical line passing through the AC) to the z-axis, and the midsagittal plane to the y-z plane. The AC-PC line was determined to pass through the superior edge of the AC and the inferior edge of the PC. The VAC line was determined to pass through the posterior margin of the AC in a vertical direction, and to be perpendicular to the AC-PC line. The intersection of AC-PC and VAC lines was then matched to the origin of the coordinates. On the realigned MR image, a brain-box surrounded by 6 planes of the cortical margin and a VPC (vertical line passing through PC) line was determined (13). Each face of the brain-box was decided upon so that the cerebral cortex was not seen any more in its next plain when these planes were examined outward from the origin of the images. They were named the anterior/posterior/superior/inferior/right/left cortical margin (ACM/PCM/SCM/ICM/RCM/LCM) of the brain, respectively (Table 2). The VPC line passed through the anterior margin of the PC, and it was perpendicular to the AC-PC line.
To explore the effects of age, gender, and age-by-gender interactions on each of the lengths defined in Table 2, an independent analysis of covariance (ANCOVA) was performed for each length with sex as the independent variable (the grouping factor), age as the covariate, and the length as the dependent variable.
For each gender, the same target brain was used for both the young/midlife and elderly groups since the global hemispheric features were not different between the young/midlife and the elderly groups, although they were different between men and women (see Results for further details). The mean values of those 7 lengths, defined in Table 2, were calculated for each gender, and the deviation from the mean values (the sum of squares of difference between the mean values and individual) were calculated for every subject. Ten subjects who had the lowest deviation from the mean distances were selected as candidates for the target brain.
Second, the basal lines and cortical peripheries were determined semi-automatically by using the image segmentation technique. To determine the cortical boundaries, the brain cortex was automatically extracted using the region growing method following morphological operations, such as dilation and erosion (17). AC, PC, AC-PC lines and the mid-sagittal plane were mannually determined from each MR image, which was then reoriented in the same way as in the first method. The length of the eleven characteristic lines between the internal landmarks and cortical boundaries were then determined. Lengths of the anterior and posterior brain and the intercommissural length were measured by the same way as the first method. The other eight distances were those between the superior/inferior cortical margins and the AC in a coronal plane passing through the AC, those between the superior/inferior cortical margins and the PC in a coronal plane passing through the PC, those between the left/right cortical margins and the AC in a coronal plane passing through the AC, and those between the left/right cortical margins and the PC in a coronal plane passing through the PC. Ten subjects with the lowest deviations from the mean distances were selected as candidates for the target brain as in the first method.
Among those candidates determined independently by the two methods, those who were commonly selected by both methods were considered as final candidates. Finally, an optimal target brain for each gender was selected by the consensus of 3 image specialists, who considered the age of subject (subjects older than 45 were excluded), asymmetry of the brain (a symmetrical brain was preferred), and the size of ventricle (those subjects with enlarged ventricles were excluded).
Target brains, which had been mannually realigned to have the origin at AC, AC-PC line on y-axis, and the midsagittal plane on the y-z plane were flipped in the left and right directions, according to neurological convention, and then resampled to have the pixel size of 2×2×2 mm by using the bilinear interpolation method. The resampled images were smoothed by convolution with an isotropic Gaussian kernel with 8 mm FWHM.
The other MR images were spatially normalized by linear (affine) transformation to the target brains and normalization parameters were reapplied to the PET images using SPM99 program. The Sinc interpolation method was used for the reslicing of images. Averaging the spatially normalized images after intensity normalization by proportional scaling created the MRI/PET templates for each age and gender. For the intensity normalization, only the brain regions were extracted from the spatially normalized individual MR images, and the mean values for the pixels inside the brain regions were calculated. Each image was divided by the mean value and then multiplied by an arbitrary value of 100 so that the mean intensity of the brain regions in the intensity-normalized image should be 100.
The mean values for the 7 global hemispheric features measured to select optimal target brain and defined in Table 2 are shown in Table 3. All the lengths in men were longer than in women. No significant age effect or age-by-gender interactions were observed for any of the lengths when ANCOVAs were employed for testing. Significant gender differences were observed in all the lengths (p<0.05, corrected for multiple comparison). The root mean square deviation between mean values and individual values for each gender was, on average, 7.31±2.56 mm (for a man) and 6.67±3.59 mm (for a woman).
Six men and six women were selected as candidates for the target brain by both of the two methods. Among them, a 34-yr old man and a 41 yr-old woman were selected as standard persons who had the target brains for the man and woman standard templates, respectively.
The overall length (ACM to PCM), height (ICM to SPM) and width (LCM to RCM) of those standard brains are shown in Table 4, and they are compared to those of MNI standard brain template and a brain of Talairach atlas. Those values for the MNI template (pixel size=1×1×1 mm) were estimated in the same way as was used in the first method to select the target brains for the Korean standard templates.
Anterior-posterior lengths of the Korean standard man and woman templates were shorter in the anterior-posterior direction than for the MNI template and the Talairach atlas. The height of Korean standard man template was smaller than the height on the MNI template, and that of the Korean woman template was slightly smaller than that of the Talairach atlas. Left-right widths of Korean man and woman templates were equivalent to those widths of the MNI template and the Talairach atlas, respectively. The smaller length and height of the Korean standard man and woman template versus the MNI template and the Talairach atlas may reflect the differences in global hemispheric shape and size between Asians and Caucasians. Length, height and width of Korean standard man template were 90%, 91% and 101%, respectively, of the MNI template, and those of woman template were 90%, 96% and 100%, respectively, of the Talairach atlas (Fig. 2).
Besides of the global differences in shape and size between male and female templates, the young/midlife and elderly templates showed differences for regional morphology and metabolism. MRI templates for elderly subjects featured ventricular enlargement, and interhemisheric and sulcal widening compared to the template for young/midlife subjects. The volume of the caudate nucleus and hippocampal formation were also smaller in the elderly MRI template.
F-18-FDG PET templates showed the metabolic differences between young and elderly subjects (Fig. 3). Compared to young/midlife templates, the elderly templates featured decreased glucose metabolism in the frontal and temporal cortex, anterior cingulate cortex, insular cortex, head of caudate, medial thalami, and cerebellum for both genders. Gender differences in template images could not determined by a visual inspection.
In this study, the hemispheric features of Korean male and female volunteers were measured, and representative brains with only minimal deviation from the average were selected as targets for composing age- and gender-specific standard templates.
The male and female subjects were only subdivided into two age groups (young/midlife and elderly). A project for the collection of image data of Korean normal volunteers is still ongoing by our group (Korean Consortium for Brain Mapping), and the larger dataset acquired through this project warrants further development of age-specific templates with a narrower range of age than was used in this study, including childhood and adolescent templates. The strategies used in this study for the selection of the target brain and composing and comparing the templates could also be applied to the currently available ICBM dataset, which can be queried using a combination of demographic and image-related attributes (12, http://www.loni.ucla.edu/ICBM/). Although we selected optimal target brains, to which the other brain MR images were all matched, other strategies have been recently suggested, whereby any of the image could have served as a target and the image shape as well as the intensity could be averaged (18, 19).
Although there are questions as to whether or not the different templates will give different findings for brain mapping studies and this should be answered in subsequent studies, the age, gender and ethnic specific brain MRI and PET templates developed in this study are anticipated as providing the framework for more accurate stereotactic standardization and anatomical localization. Especially, these data would be useful for the analysis of brain MRI and PET data of the Far East Asian people, such as Japanese and Chinese, who have a cultural and genetic similarity with Koreans. The standard templates, probabilistic labeling information and quantification program developed in this study is available on request.
Figures and Tables
 | Fig. 1Flowchart for the strategy used in this study to select optimal target brains. Two different methods were independently used to determine 10 male and 10 female candidates, and those who were commonly selected by both methods were considered as a final candidate. The final target brain for each gender was selected with the consensus of 3 image specialists by considering the age of the subject, asymmetry of brain and size of the ventricles. |
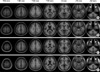 | Fig. 2T1-SPGR MRI templates. (A) Young/midlife man. (B) Elderly man. (C) Young/midlife woman. (D) Elderly woman. |
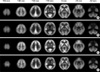 | Fig. 3F-18-FDG PET templates. (A) Young/midlife man. (B) Elderly man. (C) Young/midlife woman. (D) Elderly woman. |
Table 1
Demographic information of the subject groups, and numbers of MRI and PET scans performed for the subjects in each group

References
1. Kasai K, Iwanami A, Yamasue H, Kuroki N, Nakagome K, Fukuda M. Neuroanatomy and neurophysiology in schizophrenia. Neurosci Res. 2002. 43:93–110.

2. Demetriades AK. Functional neuroimaging in Alzheimer's type dementia. J Neurol Sci. 2002. 203-204:247–251.

3. Theodore WH, Gaillard WD. Neuroimaging and the progression of epilepsy. Prog Brain Res. 2002. 135:305–313.

4. Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002. 159:1642–1652.

5. Munte TF, Altenmuller E, Jancke L. The musician's brain as a model of neuroplasticity. Nat Rev Neurosci. 2002. 3:473–478.

6. Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS. Cross-modal plasticity and cochlear implants. Nature. 2001. 409:149–150.

7. Giraud AL, Truy E, Frackowiak R. Imaging plasticity in cochlear implant patients. Audiol Neurootol. 2001. 6:381–393.

8. Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. Proc IEEE Nucl Science Symp Medl Imaging Conf. 1993. In : Nuclear Science Symposium and Medical Imaging Conference; 10/31/1993 - 11/06/1993; San Francisco, CA, USA. 1813–1817.

9. Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999. 7:254–266.

10. Lancaster JL, Fox PT, Downs H, Nickerson DS, Hander TA, El Mallah M, Kochunov PV, Zamarripa F. Global spatial normalization of human brain using convex hulls. J Nucl Med. 1999. 40:942–955.
12. Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Camon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci. 2001. 356:1293–1322.

13. Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system-an approach to cerebral imaging. 1988. New York: Thieme Medical Publishers.
14. Fox PT, Perlmutter JS, Raichle ME. A stereotactic method of anatomical localization for positron emission tomography. J Comput Assist Tomogr. 1985. 9:141–153.

15. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995. 2:189–210.

16. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002. 15:273–289.

17. Yoon U, Lee JM, Kim JJ, Lee SM, Kim IY, Kwon JS, Kim SI. Modified magnetic resonance image based parcellation method for cerebral cortex using successive fuzzy clustering and boundary detection. Ann Biomed Eng. 2003. 31:441–447.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download