Abstract
Repair of skeletal defects with vascularized bone grafts has many advantages over non-vascularized free grafts, but the availability of these grafts is extremely limited. This study was designed to determine whether new vascularized bone could be engineered by transplantation of osteoblasts around existing vascular pedicles using biodegradable, synthetic polymer as a cell delivery vehicle. Cells were isolated from the periosteum of fetal bovine humerus, and then seeded onto non-woven multifilament, polyglycolic acid polymer. The polymers provided three dimensional support during in vitro culture. The cell-polymer constructs were maintained in vitro for two weeks and then implanted around the right femoral vessels of twelve athymic nude rats. The polymer templates without the cells were implanted around left femoral vessels of each mouse as a control. Twelve rats were sacrificed at the following intervals: three rats at six,and nine rats at nine weeks. New bone formation was evident in 10 out of the 12 periosteal-derived cell seeded implants. At six weeks, the tissue was primarily composed of what appeared both grossly and histologically to be cartilage enveloping small islands of osteoid. The degree of osteoid and bone formation progressed with time, as blood vessels invaded the tissue. This tissue ultimately underwent morphogenesis to become an organized trabeculated bone with a vascular pedicle. We believe that this technique may prove to be useful in the reconstruction of bony defect.
Skeletal defects, derived from traumatic, degenerative, or tumorous causes, represent a major challenge to reconstructive surgery. For more complex bony defects, the conventional iliac crest non-vascularized corticocancellous free bone grafts have limited application and success. Despite their superiority as bone replacements and refinements in surgical technique, vascularized bone grafts or bone flaps, on the other hand, have not attained the popularity of soft tissue flaps. This is in part due to the limited donor tissue, donor site morbidity, complexity of the surgery, inability to shape the graft to the exact configuration of the bony defects and the availability of alternate. Alternative methods of skeletal reconstruction include the use of alloplastic materials or synthetic implants.
Since the early 1990s, significant progress has been made in constructing new tissue using cell transplantation. Transplantation of cells, instead of entire pieces of tissue, presents the opportunity to expand a small donor population into a larger transplantable mass. Furthermore, use of biodegradable polymer to deliver the cells and act as a templates for tissue regeneration may allow completely natural bone and cartilage (1, 2). Recently, there are many reports on bone engineering using periosteal derived cell as a cell source and various cell delivery vehicles such as a tricalcium phosphate (α-TCP), hydroxyapatitie (HA), polylactico-glycolic acid (PLGA) in Hutmachker's review (3). And also, prefabrication of vascularized bone graft using oseoconductive scaffold without cell transplantation have been reported (4).
We now report the construction of bone using periosteum derived cell-polymer constructs placed around existing vascular pedicles.
Osteoblasts were isolated as described by Koshihara et al. (5). Periosteum was obtained from the humerus of newborn calf shoulders within 6 hr of sacrifice. Shoulders were rinsed in 10% povidone-iodine under sterile conditions, and the soft tissue was removed by sharp dissection. Periosteum was cut into pieces a 2×2 cm, rinsed in phosphate buffered saline (PBS), and placed into 35 mm tissue culture dishes (Costar: Cambridge, MA, U.S.A.). Each dish contains 2 mL of Media 199 (Gibco: Grand Island, NY, U.S.A.) supplemented with 10% fetal calf serum, L-glutamine (292 µg/mL), penicillin (100 U/mL), streptomycin (100 µg/mL), ascorbic acid (5 µg/mL) and Vitamin D3 (40 ng/mL). The specimens were incubated at 37℃ in the presence of 5% CO2 for 2 weeks to allow cells to migrate and proliferate in monolayer on the bottom of each well. One layer of cells was transferred onto a non-woven mesh of 15 µm polyglycolic acid fibers (Davis and Geck, Danbury, CT, U.S.A.), size of 5×7×1 mm. Cells adhered to and grew over the polymer mesh over the ensuing 7-10 days to form a multilayer of cells along the majority of the polymer fibers. Samples of the culture medium were analyzed for the presence of osteocalcin using a radioimmunoassay kit (Biomedical Technologies Inc. Stoughton, MA, U.S.A.). The results of this analysis were normalized for cell number by quantitating the number of cells adherent to the polymer scaffold. Culture media was exchanged once every 3 days.
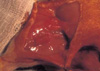
Cell-polymer devices were subsequently transplanted into athymic nude rats that weigh an average of 250-300 g under general anesthesia using inhalation of methoxyflurane and intramuscular injections of ketamine. Inguinal areas were washed with 10% povidone-iodine solution, and under sterile conditions, the femoral vessels of animals were exposed through a longitudinal skin incision under a surgical microscope. The polymer construct seeded with cells was wrapped around the right femoral vessel (experimental), and fixed in place with 6-0 nylon (Ethicon Inc. Piscataway, NJ, U.S.A.) suture (Fig. 1). The left femoral vessels were wrapped with polymer templates without cells (control). Three rats were sacrificed at 6 weeks, and nine rats were sacrificed at 9 weeks. Excised specimens were evaluated grossly and histologically by cutting 3 µm sections and staining with hematolxylin and eosin.
Cells readily adhered to the polymer fibers in multiple layers. To confirm that the isolated cells were osteoblasts, the secretion of osteocalcin, a protein specifically secreted by osteoblast, into the culture medium was quantitated. Osteocalcin was present in concentration similar to that previously reported (5) and concentration was upregulated by addition of vitamin D to the culture medium (Table 1).
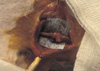
To assess the ability of these constructs to form vascularized bone tissue, they were subsequently implanted around the femoral vessels of athymic rats. Each polymer construct contained approximated 2×106 cells at the time of implantation. Examination of excised specimens revealed progressive bone formation in the implants seeded with osteoblast (Fig. 2). The in vivo results are summarized in Table 2 and presented briefly below. Histological examination of the specimens revealed that progressive bone formation was found in 10 of 12 implants seeded with osteoblasts. Specimens at 6 weeks were primarily composed of cartilage with islands of osteoid tissue seen peripherally associated with blood vessel invasion (Fig. 3). At 9 weeks, the new bone was organized in that trabeculae were formed, and the bone appeared lamellar when sections were viewed using light microscopy (Fig. 4). However, control polymer devices implanted without cells did not result in bone formation over the entire time course. Grossly, there was no reminant of polyglycolic acid fiber in control side (left).
Free bone grafts are commonly used in reconstructive surgery. Use of vascularized bone grafts increase survival and minimize bone resorption. Experimental and clinical studies indicate that immediately vascularized autografts improved osteocyte survival and enhanced bone incorporation (6, 7). Thus, vascularized bone grafts or flaps are useful for patients with the poor recipient bed either scarred or irradiated.
To circumvent these limitations, our laboratory has been attempting to construct new structural tissues, such as bone and cartilage using cell transplantation (1, 8). By using synthetic biodegradable polymers as cell delivery vehicle it would be possible to engineer new, mature cartilage (9) and bone tissue in specific shapes and geometric design in near future. We have found that it is possible to engineer a vascularized bone by transplanting periosteum-derived cells around the pre-existing vascular pedicle using a biodegradable polymer as the cell delivery device. Degradation of the polymer over time leads to a nearly complete natural tissue, composed of autologous cells embedded within their own matrix and devoid of foreign materials. A central question arising from these results is whether the transplanted cell-polymer devices developed into bone tissue, or whether they induced host cells to migrate to the site and develop into bone tissue (10). The transplanted cells phenotypically resemble osteoblasts before transplantation, and critically, control polymers implanted without these cells induced no bone formation. These results suggest to us that the implanted cells were responsible for the new bone formation.
The results of these studies indicate that it is possible to create a vascularized bone tissue suitable for reconstruction using cells seeded on biodegradable polymer templates. Expansion of a limited source is possible with this technique, and this approach opens up the possibility of autologous cell transplants to reconstruct bony defects with vascularized bone grafts.
Figures and Tables
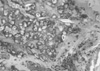 | Fig. 3Histologic section of implant removed at 6 weeks reveals that blood vessels are found in the newly created tissue along with osteoid formation (H&E, ×100). |
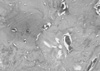 | Fig. 4Histologic section of implant removed at 9 weeks. Lamellar-like calcified bone tissue is observed along with a cluster of cells. |
References
1. Vacanti CA, Kim W, Upton J, Vacanti MP, Mooney D, Schloo B, Vacanti JP. Tissue-engineered growth of bone and cartilage. Transplant Proc. 1993. 25:1019–1021.
2. Yoon TR, Jung EJ, Seo HY, Na SJ. In vitro and in vivo osteogenic activity of rabbit periosteum and periosteal cells. J Korean Orthop Res Soc. 2003. 6:238–249.
3. Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003. 9:Suppl 1. S45–S63.

4. Terheyden H, Knak C, Jepsen S, Palmie S, Rueger DR. Mandibular reconstruction with a prefabricated vascularized bone graft using recombinant human osteogenic protein-1. Int J Oral Maxillofac Surg. 2001. 30:373–379.
5. Koshihara Y, Kawamura M, Endo S, Tsutsumi C, Kodama H, Oda H, Higaki S. Establishment of human osteoblastic cells derived from periosteum in culture. In Vitro Cell Dev Biol. 1989. 25:37–43.

6. Berggren A, Weiland AJ, Dorfman H. Free vascularized bone grafts: Factors affecting their survival and ability to heal to recipient bone defects. Plast Reconstr Surg. 1982. 69:19–29.
7. Weiland AJ, Phillips TW, Randolph MA. Radiologic, histologic and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast Reconstr Surg. 1984. 74:368–379.
8. Vacanti CA, Langer R, Schloo B, Vacanti JP. Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plast Reconstr Surg. 1991. 88:753–759.

9. Kim WS, Vacanti JP, Cima L, Mooney D, Upton J, Puelacher WC, Vacanti CA. Cartilage engineered in predetermined shapes employing cell transplantation on synthetic biodegradable polymers. Plast Reconstr Surg. 1994. 94:233–237.

10. Mulliken JB, Glowacki J. Induced osteogenesis for repair and construction in craniofacial region. Plast Reconstr Surg. 1980. 65:553–560.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download