Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune disease of unknown etiology. We studied the diagnostic performances of anti-cyclic citrullinated peptides antibody (anti-CCP) assay and recombinant anti-citrullinated filaggrin antibody (AFA) assay by enzyme linked immunosorbent assay (ELISA) in patients with RA in Korea. Diagnostic performances of the anti-CCP assay and AFA assay were compared with that of rheumatoid factor (RF) latex fixation test. RF, anti-CCP, and AFA assays were performed in 324 RA patients, 251 control patients, and 286 healthy subjects. The optimal cut off values of each assay were determined at the maximal point of area under the curve by receiver-operator characteristics (ROC) curve. Sensitivity (72.8%) and specificity (92.0%) of anti-CCP were better than those of AFA (70.3%, 70.5%), respectively. The diagnostic performance of RF showed a sensitivity of 80.6% and a specificity of 78.5%. Anti-CCP and AFA showed positivity in 23.8% and 17.3% of seronegative RA patients, respectively. In conclusion, we consider that anti-CCP could be very useful serological assay for the diagnosis of RA, because anti-CCP revealed higher diagnostic specificity than RF and AFA at the optimal cut off values and could be performed by easy, convenient ELISA method.
Rheumatoid arthritis (RA) is a systemic autoimmune disease of unknown etiology with chronic joint inflammation. The major autoantibody detected in RA patients is rheumatoid factor (RF). RF positivity is included in the American College of Rheumatology (ACR) (1) classification criteria for the diagnosis of RA. RF assay is an easy, convenient method using automatic instruments. However, since RF is detected in only 50-80% of RA sera (2) and frequently present in patients with other autoimmune disease and in the elderly healthy population (3), diagnosis of RA using RF assay remains suboptimal. Other autoantibodies detected in RA are anti-RA33, anti-Sa, anti-p68, anti-calpastatin, and antiperinuclear factor (2), but these antoantibodies have demonstrated lower sensitivity for the diagnosis of RA.
Among autoantibodies found in RA, the antiperinuclear factor (APF) that recognizes perinuclear granules in human buccal keratinocytes (4), and the antikeratin antibody (AKA) that recognizes water-soluble acidic-neutral isoforms of filaggrin in the human epidermis (5) and various molecular forms of (pro) filaggrin in the rat esophagus epithelium (6), were known to be the most specific antibodies in RA. APF and AKA, which had been considered as different antibodies at first, were recognized to belong to the same autoantibody family directed to (pro) filaggrin, so both of them are called antifilaggrin autoantibodies at present (7, 8). Vincent et al. reported that the sensitivity of APF was 67% and a specificity was 93%, and AKA showed a sensitivity of 52% and a specificity was 97% (9) in RA patients. However, performance of APF assay was restricted to a few specialized laboratories because the technique was subjective and laborious, because of the necessity to use preselected buccal cell donors, and the problematic interlaboratory standardization (2).
Recently, Schellekens et al. discovered that antibodies specific for RA containing citrulline residues were the same as those defined as APF and AKA, and were detected in over 80% of RA sera using several synthetic peptides (10). These citrullinated peptides are formed by post-translational modification of arginine residues to citrulline residues by the enzyme peptidyl-arginine deiminase (11). The antibodies to these citrullinated peptides such as anti-cyclic citrullinated peptides antibodies (anti-CCP) and anti-filaggrin antibodies (AFA) have been reported to be highly specific for RA (2, 12). These new serological tests have advantage of easy performance by convenient enzyme linked immunosorbent assay (ELISA) method.
We studied the diagnostic performances of anti-CCP and AFA by ELISA method and compared those with the diagnostic performance of RF. We also evaluated whether anti-CCP and AFA were useful serological tools to diagnose RA in Korean patients.
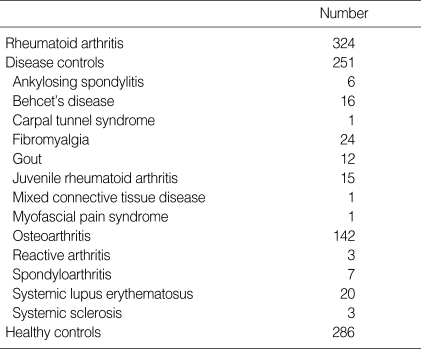
Serum samples were obtained from 324 patients diagnosed as RA according to the ACR criteria (1), and from 251 outpatients with other rheumatic disease from the department of Rheumatology. 286 healthy subjects were considered as control group and all the samples were screened by comprehensive Medical testing from the Department of Family Medicine at Eulji University Hospital from January to May, 2002. The serum samples were aliquoted and stored at -70℃ until assayed. Patient's charts were reviewed for demographic information, clinical diagnoses, and other laboratory data. Sex ratio was 49 males (15.1%) and 275 females (84.9%) in RA patients and 41 males (16.3%) and 210 females (83.7%) in other rheumatic diseases. RA patients showed 14.6 (2-41) years in disease duration and had been treated with various disease modifying antirheumatic drug (DMARD). Other rheumatic diseases included osteoarthritis (n=142), fibromyalgia (n=24), systemic lupus erythematosus (SLE) (n=20), Behcet's disease (n=16), juvenile RA (n=15), gout (n=12), spondyloarthritis (n=7), ankylosing spondyloarthritis (n=6), systemic sclerosis (n=3), reactive arthritis (n=3), carpal tunnel syndrome (n=1), mixed connective tissue disease (n=1), and myofascial syndrome (n=1) (Table 1).
Anti-CCP assay was determined by using highly purified synthetic cyclic citrullinated peptides as substrates. AFA assay was performed using the recombinant human filaggrin containing citrulline residues after in vitro deimination. Either anti-CCP or AFA was determined by the ELISA method (Tohso, Tokyo, Japan) according to the manuals. RF was measured by the latex fixation test by Hitachi 7170 (Hitachi Co, Tokyo, Japan). We used DIASTAT anti-CCP kit (MBL Co., Nagoya, Japan) for the measurement of anti-CCP, and anti-citrullinated filaggrin ELISA kit (MBL Co., Nagoya, Japan) for AFA. Control sera were included on all plates to monitor plate to plate variation. Variation never exceeded 5% and values were not corrected. The optimal cut off values of each tests were determined at the maximal point of area under the curve by receiver-operator characteristics (ROC) curve and we determined and compared the diagnostic performances among anti-CCP, AFA, and RF.
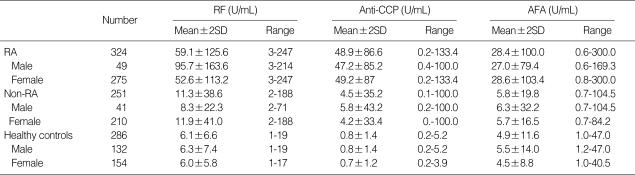
Total number of healthy subjects was 286 (132 male, 154 female) with a mean age of 50.4 yr (16-72 yr). The range of anti-CCP, AFA, and RF was 0.2-5.2 U/mL, 1.0-47.0 U/mL, and 1-19 U/mL, respectively. All tests showed no significant differences in the mean value between male and female (Table 2). The positive numbers of anti-CCP, AFA, and RF were 3 (1.3%), 73 (25.5%), and 42 (14.7%) at the optimal cut off values, respectively. The cut off value of anti-CCP, AFA, and RF was 3.8 U/mL, 4.5 U/mL, and 9 U/mL, respectively. Healthy subjects with anti-CCP positivity did not have any other rheumatic disease.
The male:female ratio was 1:5.6 with a mean age of 51 yr (22-83 yr) in RA patients. The range of anti-CCP titers in RA was 0.2-133.4 U/mL and showed no significant difference in the mean value between male and female (p=0.761) (Table 2). The range of AFA titers was 0.6-300 U/mL and showed no significant difference in the mean value between male and female (p=0.832). The male:female ratio was 1:5.1 with a mean age of 53.4 yr (4-90 yr) in disease control group. The range of anti-CCP and AFA titers were 0.1-100.0 U/mL and 0.7-104.5 U/mL in the patients with other rheumatic disease, respectively. The range of RF titers was 3-247 U/mL and revealed a significant difference between male (95.7 U/mL) and female (52.6 U/mL) in the mean value in RA (p=0.001). The range of RF titers was 2-188 U/mL in the patients with other rheumatic disease. Our results showed significant difference in the anti-CCP, AFA, RF titer between RA patients and disease control group (p=0.000).
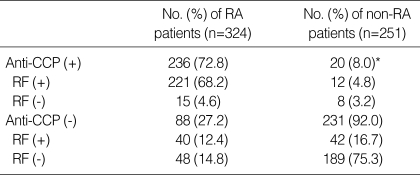
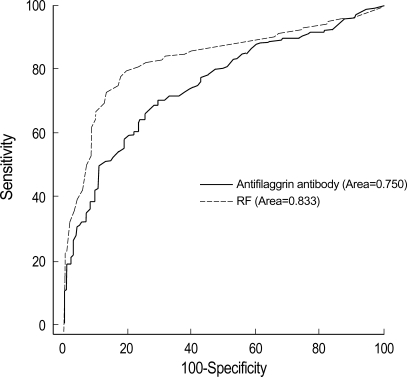
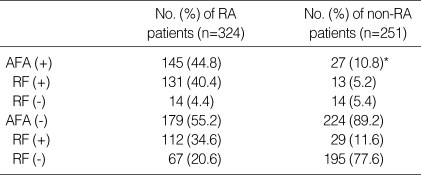
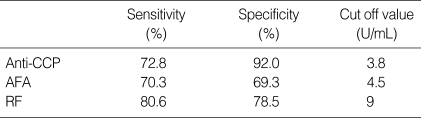
The cut off value of anti-CCP, AFA, and RF was 3.8 U/mL, 4.5 U/mL, 9 U/mL (Table 5). Anti-CCP showed 72.8% of sensitivity and 92% of specificity (Table 3, 5). The false positivity of anti-CCP assay in RA was 8.0% and following diseases were included; Behcet's disease (n=1), fibromyalgia (n=1), gout (n=2), juvenile rheumatoid arthritis (n=6), osteoarthritis (n=6), reactive arthritis (n=1), spondyloarthritis (n=2), and SLE (n=1) (Table 3). Anti-CCP positivity was 23.8% among sero-negative RA patients. AFA showed 70.3% of sensitivity and 69.3% of specificity (Table 4, 5). The false positivity of AFA in RA was 10.8% and following diseases were included; Behcet's disease (n=1), fibromyalgia (n=3), gout (n=1), juvenile rheumatoid arthritis (n=4), osteoarthritis (n=10), spondyloarthritis (n=1), SLE (n=6), and systemic sclerosis (n=1) (Table 4). AFA positivity was 17.3% among seronegative RA patients. RF showed 80.6% of sensitivity and 78.5% of specificities (Table 5). The anti-CCP assay showed better specificity than other tests, and these results were consistent with those of previous studies (2, 9, 13). Diagnostic accuracy among anti-CCP, AFA, and RF assays by ROC curve was compared and the area under the curve of the anti-CCP, AFA, and RF were 0.837, 0.750, and 0.833, respectively. Diagnostic accuracy between the anti-CCP and RF was not significantly different (p=0.857), but that between AFA and RF was significantly different (p=0.000) (Fig. 1, 2).
The modern trend of RA treatment has been changed to start treatment as early as possible, based on the concept that early control of inflammation results in reduced joint damage (14). The RF assay has known to have suboptimal sensitivity and specificity for the diagnosis of RA. Therefore, easier, more convenient, higher specific serological methods have been required for the diagnosis of RA.
Recently, anti-CCP and AFA assays by ELISA method were developed using the synthetic cyclic citrullinated peptide and the recombinant anti-citrullinated filaggrin as substrates for the diagnosis of RA, which was reported highly specific and significant (2, 9). The native recombinant filaggrins do not react with antifilaggrin autoantibodies, however, after in vitro deimination, recombinant filaggrins become immunoreactive (15). Schellekens et al. reported that synthetic peptides were, in principle, ideal antigenic substrates and peptides with a predefined sequence could be produced in large quantities with a high degree of purity, and their relative small size reduced the chance of nonspecific interactions occurring with other serum components (2).
In this study, we evaluated the diagnostic performances of anti-CCP and AFA assay by ELISA method as useful new serological tests for the diagnosis of RA in 324 RA patients, 259 patients with other rheumatic disease and 286 healthy subjects. In 286 healthy subjects, the number of anti-CCP, AFA, RF positivity was 3 (1.0%), 73 (25.5%), 42 (14.7%) at the optimal cut off values, respectively. Healthy subjects with anti-CCP reactivity did not have any other rheumatic disease and showed low titer of anti-CCP reactivity. We considered that the anti-CCP assay had higher specificity than other assays in healthy population. In RA patients, sex ratio (M:F) is 1:5.6 and the mean value of anti-CCP between male and female had no significant difference in RA (p=0.761). The diagnostic performance of anti-CCP and AFA in sera obtained from patients with RA and other rheumatic disease showed the sensitivity of 72.8%, 70.3% and the specificity of 92.0%, 69.3% at the optimal cut off values, respectively. Schellekens et al. reported that ELISA methods using the cyclic citrullinated peptide (CCP-ELISA), in a series of 149 RA and 312 control sera, had a diagnostic sensitivity of 48% for a specificity of 96% (2). Kroot et al. identified 66% positive RA serum samples with CCP-ELISA (3). In this study, the diagnostic sensitivity proposed for CCP-ELISA showed better than that of other studies, and slightly lower specificity than that of Schellekens et al. (2). However, we considered that our study did not include healthy subjects in control group and showed a slightly lower specificity of 92.0% compared to 96% specificity by Schellekens et al. We concluded that anti-CCP showed high specificity for the diagnosis of RA.
Nogueira et al. reported the diagnostic sensitivity of 52% for a specificity of 95% with AFA-ELISA-rec (using the recombinant human filaggrin deiminated in vitro) and sensitivity of 51% for a specificity of 95% with AKA (12). Palosuo et al. developed ELISA methods for detection of the antifilaggrin autoantibodies using purified human filaggrin and proposed a sensitivity of 71% for a specificity of 95% (16). Aho et al. reported 49% positive RA serum samples with their AhFA-ELISA vs. 47% with APF (17). Vincent et al. reported that in a series of 279 RA patients and 213 control patients, APF had a sensitivity of 67% and a specificity of 93% and at 93% specificity, was better than AKA (57%) and AFA-immuno-blotting (57%) (9). However, APF at 99% specificity was lower sensitivity of 30% (9). In this study, the AFA assay was lower specificity than other studies, however, it was as similar as the sensitivity of APF. We considered that AFA might have lower sensitivity, because AFA used a recombinant protein as target antigens and its recombinant filaggrin corresponded to only one filaggrin polypeptide of several filaggrin polypeptides (18).
The range of RF was 3-247 U/mL and the mean value of RF had a significant difference between male and female in RA patients (p=0.001) (Table 2). We thought there was no reported data about difference of mean value between male and female in RA patients and we needed further evaluation. The cut off value of RF was 9 U/mL and its sensitivity and specificity was 80.6% and 78.5%. These results showed higher sensitivity and lower specificity than those of Visser et al. using the latex fixation test (sensitivity of 65.6%, specificity of 90.9%) (13). Vittecoq et al. proposed that among ACR criteria, only RF detected by agglutination tests was taken into account, however, the measurement of IgM-RF by ELISA, in addition to latex fixation test, could increase the number of RF-positive cases in early RA (19). Visser et al. showed that the diagnostic characteristics of the ELISAs for IgG and IgA RF in discriminating RA from non-RA were poor and the test characteristics of the ELISA for IgM RF and the latex fixation test proved to be much better, and compared reasonably well to each other, though the specificity of the latex fixation test was greater than that of the ELISA for IgM (13). Schellekens et al. reported the sensitivity of 54% and specificity of 91% by IgM-RF ELISA (2). Visser et al. showed the sensitivity of 69.2% and the specificity of 86.0% by IgM-RF ELISA, and suggested that greater sensitivity and specificity of the latex fixation test can be explained by differences in patient's selection, criteria for the diagnosis of RA, and study design (13).
Our study showed that the anti-CCP and AFA were highly specific for RA and could be detected up to 23.8% and 17.3% of RF-negative sera, respectively, compared to 35% of Schellekens et al. (Table 3, 4) (2). The false positivity of anti-CCP was 8.0% and following diseases were included; Behcet's disease (n=1), fibromyalgia (n=1), gout (n=2), juvenile rheumatoid arthritis (n=6), osteoarthritis (n=6), reactive arthritis (n=1), spondyloarthritis (n=2), and SLE (n=1) (Table 4). The diagnostic accuracy among the anti-CCP, AFA, and RF was compared, and the area under the curve of anti-CCP, AFA, and RF was 0.837, 0.750, and 0.833, respectively. Diagnostic accuracy between the anti-CCP and RF was not significantly different (p=0.857), but that between AFA and RF was significantly different (p=0.000) (Fig. 1, 2). Nogueira et al. reported that the discrepancies between the performances of the tests, which may result from the composition of the series of patients analyzed and from subtle interlaboratory technical differences in performing the same test, underline the difficulties in comparing the results with each other objectively in the absence of a common standardized reference test (12).
In conclusion, anti-CCP assay by ELISA could be performed more objectively and quantitatively, and showed higher specificity than AFA and RF in patients with RA. Therefore, we propose that anti-CCP assay could be a useful new serological test for the diagnosis of Korean patients with RA.
References
1. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–324. PMID: 3358796.
2. Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000; 43:155–163. PMID: 10643712.
3. Kroot EJ, de Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, van't Hof M, van de Putte LB, van Rijswijk MH, van Venrooij WJ, van Riel PL. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000; 43:1831–1835. PMID: 10943873.
4. Nienhuis RL, Mandema E. A new serum factor in patients with rheumatoid arthritis, the antiperinuclear factor. Ann Rheum Dis. 1964; 23:302–305. PMID: 14178016.
5. Simon M, Girbal E, Sebbag M, Gomes-Daudrix V, Vincent C, Salama G, Serre G. The cytokeratin filament-aggregating protein filaggrin in the target of the so-called "antikeratin antibodies", autoantibodies specific for rheumatoid arthritis. J Clin Invest. 1993; 92:1387–1393. PMID: 7690781.
6. Girbal E, Sebbag M, Gomes-Daudrix V, Simon M, Vincent C, Serre G. Characterization of the rat oesophagus epithelium antigens defined by the so-called "antikeratin antibodies", specific for rheumatoid arthritis. Ann Rheum Dis. 1993; 52:749–757. PMID: 7504913.
7. Serre G. Autoantibodies to filaggrin/deiminated fibrin (AFA) are useful for the diagnosis and prognosis of rheumatoid arthritis, and are probably involved in the pathophysiology of the disease. Joint Bone Spine. 2001; 68:103–105. PMID: 11324923.
8. Sebbag M, Simmon M, Vincent C, Masson-Bessiere C, Girbal E, Durieux JJ, Serre G. The antiperinuclear factor and the so-called "antikeratin antibodies" are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1995; 95:2672–2679. PMID: 7539459.
9. Vincent C, de Keyser F, Masson-Bessiere C, Sebbag M, Veys EM, Serre G. Antiperinuclear factor compared with the so called "antikeratin" antibodies and antibodies to human epidermis filaggrin, in the diagnosis of arthritides. Ann Rheum Dis. 1999; 58:42–48. PMID: 10343539.
10. Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constitute of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998; 101:273–281. PMID: 9421490.
11. Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase: substrate specificity and structural relationship of the natural substrates trichohyalin and filaggrin. J Biol Chem. 1996; 271:30709–30716. PMID: 8940048.
12. Nogueira L, Sebbag M, Vincent C, Arnaud M, Fournié B, Cantagrel A, Jolivet M, Serre G. Performance of two ELISAs for antifilaggrin autoantibodies, using either affinity purified or deiminated recombinant human filaggrin, in the diagnosis of rheumatoid arthritis. Ann Rheum Dis. 2001; 60:882–887. PMID: 11502616.
13. Visser H, Gelinck LB, Kampfraath AH, Breedveld FC, Hazes JM. Diagnostic and prognostic characteristics of the enzyme linked immunosorbent rheumatoid factor assays in rheumatoid arthritis. Ann Rheum Dis. 1996; 55:157–161. PMID: 8712877.
14. Emery P. The optimal management of early rheumatoid disease: the key to preventing disability. Br J Rheumatol. 1994; 33:765–768. PMID: 8055205.
15. Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, Simon M, Senshu T, Masson-Bessière C, Joliver-Reynaud C. The epitopes targeted by the rheumatoid arthritis-associated anti-filaggrin autoantibodies are post-translationally generated on various sites of (pro) filaggrin by deimination of arginine residues. J Immunol. 1999; 162:585–594. PMID: 9886436.
16. Palosuo T, Lukka M, Alenius H, Kalkkinen N, Aho K, Kurki P, Heikkilä R, Nykänen M, von Essen R. Purification of filaggrin from human epidermis and measurement of antifilaggrin autoantibodies in sera from patients with rheumatoid arthritis by an enzyme-linked immunosorbent assay. Int Arch Allergy Immunol. 1998; 115:294–302. PMID: 9566352.
17. Aho K, Palosuo T, Lukka M, Kurki P, Isomaki H, Kautiainen H, von Essen R. Antifilaggrin antibodies in recent-onset arthritis. Scand J Rheumatol. 1999; 28:113–116. PMID: 10229141.
18. Harding CR, Scott IR. Histidine-rich proteins (filaggrins):structural and functional heterogeneity during epidermal differentiation. J Mol Biol. 1983; 170:651–673. PMID: 6195345.
19. Vittecoq O, Jouen-Beades F, Krzanowska K, Bichon-Tauvel I, Ménard JF, Daragon A, Tron F, Le Loët X. Rheumatoid factors, anti-filaggrin antibodies and low in vitro interleukin-2 and interferon-γ production are useful immunological markers for early diagnosis of community cases of rheumatoid arthritis. A preliminary study. Joint Bone Spine. 2001; 68:144–153. PMID: 11324930.
Fig. 1
Receiver operator characteristics (ROC) curves of anti-CCP and RF with or without RA (p=0.857).
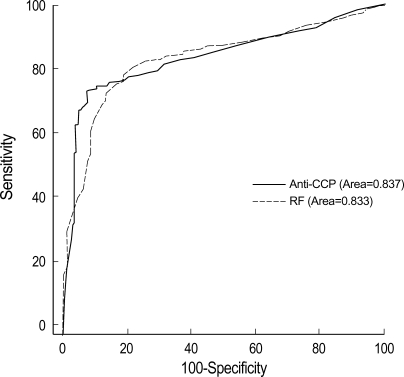
Table 3
Test results at the optimal cut off value for anti-CCP and RF in patients with and without RA




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download