Abstract
We detected pregnancy related new molecule, human chorionic gonadotropin related protein (hCGRP) in the urine of a pregnant women by using a monoclonal antibody against the human chorionic gonadotropin (hCG). This study examined the effectiveness of urinary hCGRP quantification in diagnosing ectopic pregnancy. This study included 40 normal pregnant women and 25 patients with ectopic pregnancy. Patients' serum and urinary intact whole hCG (i-hCG) and hCGRP concentrations were measured using sandwich ELISA and the ratio of hCGRP to i-hCG was calculated. Statistical analysis was performed using statistical package for social sciences (SPSS) 10.0. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the cut-off value to discriminate ectopic pregnancies from normal intrauterine pregnancies. Urinary hCGRP and hCGRP/i-hCG ratio in ectopic pregnancy group (14±6.6 ng/mL, 4.6±1.9%, respectively) were significantly lower than those of normal pregnancy group (149±10.2 ng/mL, 29.7±1.9%, respectively; p<0.001). Based on ROC curve analysis, a cut-off point of urinary hCGRP/i-hCG ratio <16.2% discriminated between ectopic pregnancy and normal pregnancy with a sensitivity, specificity, positive predictive value and negative predictive value of 92.0%, 90.0%, 32.6%, and 99.5%, respectively. Urinary hCGRP/i-hCG ratio measurement may be effective in diagnosing ectopic pregnancy.
Ectopic pregnancy increases mortality and morbidity of fertile women, accounting for 9% of all deaths during the first trimester of pregnancy (1, 2). Moreover, the prevalence of ectopic pregnancy is gradually increasing worldwide. In northern Europe between 1976 and 1993, the incidence was increased from 11.2 to 18.8 per 1,000 pregnancies (3). Total admission to the hospital for ectopic pregnancy was increased from 17,800 in 1970 to 88,400 in 1989, reaching 1 case per 60 pregnancies in the United States (4, 5). It was estimated the incidence of ectopic pregnancy in Korea was 1 case per 20 to 26 pregnancies, which was relatively higher than in Western countries (6, 7).
In the past, diagnosis of ectopic pregnancy based on clinical findings such as vaginal bleeding and lower abdominal pain imposed severe constraints on early detection, although it has recently become possible to detect ectopic pregnancy at an earlier stage by determination of serum human chorionic gonadotropin (hCG) levels and the use of advanced vaginal ultrasonography techniques. In spite of this progress, both hCG measurement and vaginal ultrasonography still have limited effectiveness in diagnosing ectopic pregnancy at primary care centers due to inadequate accuracy and lack of cost-effectiveness. Moreover, it is necessary to perform repeat quantification of serum hCG levels when diagnostic results are ambiguous, which may require additional time and costs.
Serum progesterone levels was suggested as a serum marker for ectopic pregnancy, however some studies showed that serum progesterone level is not effective and poor clinical modality in the differential diagnosis of normal pregnancy, ectopic pregnancy and spontaneous abortion (8, 9). Making a quick and accurate diagnosis of ectopic pregnancy is desirable, but often difficult. Delayed diagnosis can cause rupture of Fallopian tube, intra-abdominal hemorrhage, blood transfusion and emergency laparotomy. Early diagnosis and intervention either by medical or surgical treatment of ectopic pregnancy can minimize its morbidity and mortality. Particularly, minimally invasive laparoscopic surgery at an early stage can also promote conservation of a Fallopian tube and increase future fertility.
Human chorionic gonadotropin is a glycoprotein with molecular weight of 37 kDa, that is synthesized by trophoblasts during pregnancy; it consists of non-covalently bonded subunits α and β. The secretion of hCG increases during the first trimester in geometrical progression, reaching a maximum at about 10 weeks of gestation. Thereafter, it decreases until 20 weeks of gestation and then is maintained at approximately 20% of the maximal level until late-stage gestation (10). Currently, it is a routine procedure to diagnose ectopic pregnancy by a sensitive assay for hCG. It has also been reported that ectopic pregnancy can be diagnosed by measurement of urinary core fragment, a metabolic product of hCG (11).
We detected pregnancy related new molecule, human chorionic gonadotropin related protein (hCGRP) in the urine of pregnant women by using a monoclonal antibody against the hCG. In the preliminary study we found that monoclonal antibody against hCGRP showed weaker reactivity in ectopic pregnancy than normal pregnant women. This study was conducted to examine the diagnostic usefulness of hCGRP by a comparative analysis between hCGRP and intact whole hCG (i-hCG) secretion for ectopic pregnancy. We evaluated hCGRP secretion in normal pregnant women and ectopic pregnancy patients using a sandwich ELISA employing two monoclonal antibodies, HSM6042 and HSM6043, which shows specific responsiveness to hCGRP rather than i-hCG.
We enrolled patients from the outpatient clinics at the Department of Obstetrics and Gynecology, Samsung Cheil Hospital, Sungkyunkwan University School of Medicine; Kangnam Cha Medical Center, Pochon University School of Medicine and Kangsuh Mizmedi Hospital. Forty women with normal pregnancies at 4 to 14 weeks amenorrhea, confirmed on transvaginal ultrasonography, were assigned to the normal pregnancy group. Twenty-five patients with ectopic pregnancy diagnosed by laparoscopic surgery and confirmed by histologic examination were assigned to the ectopic pregnancy group. All patients were provided with the study details, including objectives and methods, and all signed written consent forms prior to enrollment in this study.
Among the patients whose intrauterine gestational sacs had been detected on ultrasonography at prenatal or infertility clinics, only those with a confirmation of fetal heart rate during follow-up were included in the normal pregnancy group. Patients who were confirmed as missed, incomplete or complete abortion during follow-up examination were excluded.
The blood and urine were collected for the normal pregnancy group when the intrauterine gestational sac is initially found through the transvaginal ultrasonography among the patients visited prenatal or infertility clinics with 4 to 14 weeks amenorrhea. In case of chemical pregnancy, sampling was withheld until confirmation of intrauterine gestational sac. Gestational age and days after last menstrual period (LMP) was estimated by gestational sac size with transvaginal ultrasonography. Mean days after LMP was 50.2±1.23 (mean±SEM) days in normal pregnancy group. For the ectopic pregnancy group, blood and urine were collected at the time of confirmation of ectopic pregnancy by laparoscopy. Gestational age was estimated by last menstrual period. Mean days after LMP was 48.6±1.65 (mean±SEM) days in ectopic pregnancy group.
Serum and urine samples were stored at -20℃ for future analysis.
For the quantitative analysis of i-hCG and hCGRP, Humasis Co. Ltd. (Gunpo, Korea) provided our group with four types of mouse anti-hCG monoclonal antibody (HSα6301, HSM6238, HSM6042, HSM6043), as well as purified hCGRP. Levels of i-hCG were analyzed according to the international criteria of the World Health Organization (WHO) (4th IS, 75/589, NIBSC, U.K.).
Two monoclonal antibodies, HSα6301 and HSM6043, were conjugated to horseradish peroxidase (HRP) using EZ-LINK™ Plus Activated Peroxidase kit (Pierce, Rockford, IL, U.S.A.), to prepare the detection antibodies.
The concentration of i-hCG was measured using HSW6238 as the capture antibody and HRP-conjugated HSα6301 as the detection antibody. HSW6238 was diluted to 10 µg/mL in coating buffer (0.1 M carbonate buffer, pH 9.5), and was dispensed in 100-µL aliquots in 96-well plates. HSW6238 was adsorbed at 4℃ for 24 hr and rinsed with a rinsing solution (0.05% Tween/PBS). Blocking solution (300 µL; 0.2% casein/PBS) was applied and reacted at room temperature for 3 hr, then rinsed to prepare the reaction plates. Samples and hCG standards were added in 100-µL aliquots and reacted at 4℃ for 2 hr, then rinsed three times. HRP-conjugated HSα6301 was dissolved in PBS to 10 µg/mL and added in 100-µL aliquots. After 2 hours at 4℃ the plates were rinsed three times again. The development reagent of the HRP substrate kit (Bio-Rad, Hercules, CA, U.S.A.) was added in 100-µL aliquots and reacted for 10 min. After 100 µL of stopping reagent (3% oxalic acid) was added to block the reaction, the absorbance was measured with an automated ELISA reader (Bio-Rad).
The concentration of hCGRP was measured using HSM6042 as the capture antibody and HRP-conjugated HSM6043 as detection antibody, following the same basic method as described above for the i-hCG ELISA. The concentration of hCG and hCGRP was expressed in ng/µL. The intra-assay coefficient of variation (CV) was 3.5%, the inter-assay CV was 8.9%, and the sensitivity was 2.0 ng/µL.
Statistical analysis was performed using statistical package for social sciences (SPSS) 10.0. Independent sample t-test was used to compare the differences between the groups. A receiver operating characteristic (ROC) curve analysis was used to estimate the predictive power of the measured variables, and to determine the hCGRP/ihCG cut-off that optimally discriminated ectopic pregnancy from normal intrauterine pregnancy.
The cut-off point was chosen to maximize sensitivity as well as specificity. As a measure for the diagnostic ability of the cut-off to predict ectopic pregnancy, the positive and negative predictive values were determined at the calculated cut-off value. The areas under the ROC curves (AUC) were calculated by Analyte-it 1.69 program (Analyte-it software, Ltd.) to evaluate the discriminatory value of the test. Unless otherwise stated, values are expressed as means±standard error of the mean. Significance was defined as p-values less than 0.05.
Clinical characteristics of the groups are compared in Table 1. The subjects were similar with respect to mean age, gravidity, parity and estimated days after LMP. Mean amenorrheic period at the time of confirmation was 50.2±1.23 days in the normal pregnancy group and 48.6±1.65 days in the ectopic pregnancy group. There was no significant difference between the two groups (p=0.49).
The standardization of the sandwich ELISAs using two different antibody combinations, HSW6238-HSα6301 and HSM6042-HSM6043, demonstrated that specific binding occurred, permitting quantification of hCG and hCGRP with linear regression analysis R square value 0.956 and 0.995, respectively (Fig. 1).
Urinary i-hCG and hCGRP had clear distribution ranges with small standard deviations that varied according to gestational week in both the normal and ectopic pregnancy groups. Both i-hCG and hCGRP concentrations were significantly lower in the ectopic pregnancy group compare to the normal pregnancy group, with a particularly marked decrease in hCGRP levels (Fig. 2A, B, p<0.001).
In the normal pregnancy group, serum concentrations of i-hCG and hCGRP varied widely, with significantly greater standard deviations than the concentrations measured in urine. However, in the ectopic pregnancy group, serum hCGRP concentration was markedly decreased and had a small standard deviation, similar to that noted for urinary concentration (Fig. 2C, D, p<0.001).
Urinary concentrations of i-hCG and hCGRP were 160±27.4 ng/mL and 14±6.6 ng/mL, respectively, in the ectopic pregnancy group, which were significantly lower than the corresponding levels of i-hCG and hCGRP, 486±19.9 ng/mL and 149±10.2 ng/mL respectively, in the normal pregnancy group (p<0.001). In particular, urinary hCGRP concentration during early ectopic pregnancy was about eleven times lower than in the normal pregnancy group, although urinary i-hCG was only three times lower than in the normal pregnancy group. Thus the difference between the two groups in hCGRP concentrations was significantly greater than the difference in i-hCG concentrations. Consequently, the ratio hCGRP/i-hCG was significantly lower in the ectopic pregnancy group, with a value of 4.6±1.9%, than in the normal pregnancy group, which had a ratio of 29.7±1.9% (Table 1, Fig. 3, p<0.001).
To determine the clinical significance of these statistical findings, a ROC curve was derived (Fig. 4). The AUC (±SE) for the ROC curves for serum hCGRP/ihCG ratio and urinary hCGRP/ihCG ratio were 0.832±0.054 and 0.916±0.042, respectively. This value represents the overall probability (91.6%) that ectopic pregnancy can be correctly identified by urinary hCGRP/ihCG ratio measurement. By analyzing the ROC curve the cut-off value giving optimal discriminatory hCGRP/ihCG ratio were found to be 16.2% in urine and 9% in serum which sensitivity and specificity for diagnosis of ectopic pregnancy was of 80%, 85% in serum and 92%, 90% in urine respectively (Table 2).
Early diagnosis of an unruptured ectopic pregnancy is essential to diminish mortality and morbidity, and for the preservation of oviducts in patients with ectopic pregnancy. Patients with ectopic pregnancy may manifest lower abdominal pain and vaginal bleeding as abnormal clinical findings during early-stage pregnancy. Ectopic pregnancy can not be diagnosed solely on the basis of these clinical symptoms. Additional studies are necessary to make a proper diagnosis of an ectopic pregnancy prior to initiation of either medical or surgical intervention. Clinical examination, transvaginal ultrasonography, quantification of serum gonadotropin and progesterone are currently utilized as its diagnostic modalities, however no single test is performed satisfactorily thus far (8, 12, 13).
In their evaluation of diagnostic methods for ectopic pregnancy, Gracia et al. reported that vaginal ultrasonography and gonadotropin quantification gave the most satisfactory results, although they concluded that new diagnostic methods for ectopic pregnancy were required since it requires an average of 36 hr to diagnose ectopic pregnancy, not to mention the resources devoted to collecting blood samples from more than half of all obstetric patients (14).
Gonadotropin consists of two non-covalently bonded subunits α and β and is released by trophoblasts during pregnancy. Subunit α (92 amino acids) is similar to the glycoprotein hormones secreted by the pituitary gland, including follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH) and luteinizing hormone (LH). Unlike the other glycoprotein hormones, subunit β (145 amino acids) has distinct traits. From an early stage of pregnancy, hCG is released in the biochemically active form in geometrical progression, reaching maximum levels at about 10 weeks of gestation, decreasing between 10 to 16 weeks of gestation, and then the level is maintained approximately one-fifth of the prior maximum. Gonadotropin released by trophoblasts has higher activity, higher molecular weight and more negative charge during the first trimester compared to that released during late-stage pregnancy; gonadotropin has more significant biochemical activity than immune activity during the first trimester (15-17). Moreover, transformation of gonadotropin isoforms, in addition to acidity changes, occurs at about 13 weeks of gestation (18).
Apparently, the hCG level is not proportional to ovarian hormone levels in all patients, playing an important role in salvaging luteinizing hormone and maintaining estrogen and progesterone during early pregnancy. Recent studies showed that the serum levels of estradiol and progesterone were lower in patients with ectopic pregnancy than in normal pregnant women (19-21). Moreover, it is inferred that luteal dysfunction is not due to ectopic pregnancy per se, but rather to derangement of trophoblastic division, since gonadotropins secreted by trophoblastic tissues from ectopic pregnancy as well as intrauterine pregnancy have equivalent immune and biochemical activities (22, 23).
Multiple hCG related molecules are present in serum and urine during pregnancy (10). These include degraded hCG molecules, hyper- and hypoglycosylated hCG, free subunits, large free subunits, fragments. Several antibody combinations are used to measure hCG concentration. Some of these antibody combinations may detect only intact hCG molecules. Some require the C-terminal segment of the β-subunit to be intact and some detect nonnicked molecules and free β-subunit.
Cole et al. reported that the level of β-core fragment (M.W.=9000) was decreased in patients with ectopic pregnancy or spontaneous abortion as compared to normal pregnant women, showing a divergent pattern of secretion as gestation progresses. Moreover, they reported that spontaneous abortion (66%) and ectopic pregnancy (92%) could be diagnosed through use of a discrimination curve, with a false-positive rate of 4% (11, 24). Borrelli et al. reported that concentrations of all forms of hCG were lower in cases of ectopic pregnancy and spontaneous miscarriage than in viable pregnancies and measurement of hCG β isoform had 100% sensitivity at a specificity of 79% for detection of ectopic pregnancy (25).
We detected pregnancy related new molecule, hCGRP in the urine of a pregnant women by using a monoclonal antibody against the hCG and confirmed that hCGRP was different from previously known multiple hCG related molecules including α subunit, β subunit, β-core fragment and hyperglycosylated hCG. We detected separate band of hCGRP from other known molecules using Western blot analysis of the hCG standard probed with mouse anti hCG monoclonal antibodies HSM6043 and HSM6042. The hCGRP is thought to be easily degraded at room temperature and shows weak immunoreactivity to common antibody combination. We believe these are the reasons why it is difficult to separate hCGRP from other known molecules.
It is assumed that hCGRP is one of several metabolic products synthesized by trophoblasts from i-hCG during pregnancy. In their study of hCG metabolic products, Cole et al. noted that various metabolic products such as nicked hCG, hyper- and hypo-glycosylated hCG, hCG missing the C-terminal extension, free α, free β, nicked free β and β-core fragment are synthesized from i-hCG; inactive forms of gonadotropin, namely nicked hCG, free β and β-core fragment, are elevated in patients with Down's syndrome and gestational trophoblastic disease. The authors postulated that the above findings are due to activation of metabolic pathways degrading active forms of gonadotropin, as well as activity of placental nicking enzymes (24).
The combination of monoclonal antibodies HSM6043 and HSM6042 in a sandwich ELISA had markedly low reactivity to i-hCG and highly specific reactivity to the hCG isoform, hCGRP (Fig. 1, R2=0.995). Moreover, we confirmed that the above antibody combination reacted to different hCG isoforms, other than the urinary β-core fragment from the study by Cole et al. on the basis of the finding that this antibody combination reacted to serum hCG isoforms, in addition to isoforms present in the urine of pregnant women.
Another combination of monoclonal antibodies, namely HSM6031 and HSM6238, specifically captures and detects i-hCG. Analysis of the secretion of i-hCG and hCGRP in both groups, using the two different combinations of monoclonal antibodies, showed that i-hCG secretion was significantly decreased in ectopic pregnancy, a finding consistent with previous studies.
We confirmed that it is more effective to analyze hCGRP/i-hCG ratio, instead of measuring i-hCG or hCGRP levels alone, as a basis for diagnosis of ectopic pregnancy, since i-hCG levels show dilutional variation among individuals, even at the same period of gestation. Use of the ratio is especially advantageous in minimizing analytical errors without adjustment with urinary creatinine.
In this study, the results indicated that the level of i-hCG was decreased and the rate of hCG isoform degradation was increased in patients with ectopic pregnancy. It is likely that the secretion of i-hCG itself was decreased due to derangement of trophoblastic division in patients with ectopic pregnancy. Moreover, hCGRP may have a molecular structure that is more easily degraded by nicking or degradation enzymes in patients with ectopic pregnancy than in normal pregnant women; it is likewise possible that hCGRP, an intermediate of i-hCG, may be degraded quickly due to increased activity of degradation enzymes.
The ELISAs for determination of urine i-hCG levels and hCGRP/i-hCG, using monoclonal antibodies that specifically recognize the structural changes of hCG isoforms and degradation products, offer better diagnostic outcomes than hCG quantification combined with ultrasonography (80% sensitivity) which is currently known as the best diagnostic method for ectopic pregnancy (13). It is also of great advantage to have a diagnostic method that is cost-effective and independent of the experience of sonography technicians. In cases where the hCGRP/i-hCG ratio fall under the cut-off values, the time required to make a diagnosis of ectopic pregnancy is relatively short as compared to conventional diagnostic methods and it does not require repeated testing.
These studies are preliminary limited to 25 ectopic and 40 normal pregnancies. The number of patients studied was relatively small to make any conclusion. The bias may exist due to exclusion of missed, incomplete or complete abortion. Further prospective studies should be conducted on large patient populations including miscarriage group to determine the clinical effectiveness of hCGRP/i-hCG measurement for diagnosis of patients with ectopic pregnancy and to elucidate more precisely the structure and physiological function of hCGRP.
Figures and Tables
Fig. 1
Specific reactivity of two anti-hCG monoclonal antibody pairs (HSα6301/HSM6238, HSM6042/HSM6043) to intact hCG.

Fig. 2
Distribution of i-hCG and hCGRP concentration in intrauterine pregnancy and ectopic pregnancy. (A) Urine of intrauterine pregnancy, (B) Urine of ectopic pregnancy, (C) Serum of intrauterine pregnancy, (D) Serum of ectopic pregnancy.
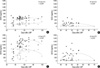
Fig. 3
Mean hCGRP/i-hCG ratios and 95% confidence intervals in intrauterine pregnancy and ectopic pregnancy group. (A) Urine samples, (B) Serum samples. IUP, intrauterine pregnancy; EP, ectopic pregnancy.

Fig. 4
Receiver operating characteristic (ROC) curve plotting sensitivity and false positive rate and the optimal discriminatory ihCG/hCGRP ratio cut-off point in serum (filled circle) and urine (filled square). ihCG, intact human chorionic gonadotropin; hCGRP, human chorionic gonadotropin related protein.

References
1. Centers for Disease Control and Prevention. Ectopic pregnancy-United States, 1988-1989. MMWR. 1992. 41:591–594.
2. Centers for Disease Control and Prevention. Ectopic pregnancy-United States, 1990-1992. MMWR. 1995. 44:46–48.
3. Storeide O, Veholmen M, Eide M, Bergsjo P, Sandvei R. The incidence of ectopic pregnancy in Hordaland County, Norway 1976-1993. Acta Obstet Gynecol Scand. 1997. 76:345–349.

4. Simms I, Rogers PA, Nicoll A. The influence of demographic change and cumulative risk of pelvic inflammatory disease on the incidence of ectopic pregnancy. Epidemiol Infect. 1997. 119:49–52.

5. Centers for Disease Control and Prevention. Ectopic pregnancy-United States, 1970-1989. MMWR. 1993. 42:73–85.
6. Cheon MJ, Kwon YI, Lew YO, Lee BH, Lee HJ, Kim CJ, Kwon DJ, Lee JW, Park TC. A clinical study of ectopic pregnancy during recent 5 years. Korean J Obst Gynecol. 2001. 44:283–289.
7. Tak BM, Kim KM, Ryu HK, Yang KS, Chung HS. A clinical study on ectopic pregnancy. Korean J Obst Gynecol. 1998. 41:819–828.

8. McCord ML, Muram D, Buster JE, Arheart KL, Stovall TG, Carson SA. Single serum progesterone as a screen for ectopic pregnancy: exchanging specificity and sensitivity to obtain optimal test performance. Fertil Steril. 1996. 66:513–516.

9. Ledger WL, Sweeting VM, Chatterjee S. Rapid diagnosis of early ectopic pregnancy in an emergency gynaecology service--are measurements of progesterone, intact and free beta human chorionic gonadotrophin helpful? Human Reprod. 1994. 9:157–160.
10. Cole LA, Kardana A, Park SY, Braunstein GD. The deactivation of hCG by nicking and dissociation. J Clin Endocrinol Metab. 1993. 73:704–710.

11. Cole LA, Isozaki T, Jones EE. Urine β-core fragment, a potential screening test for ectopic pregnancy and spontaneous abortion. Fetal Diagn Ther. 1997. 12:336–339.

12. Stovall T, Kellerman AL, Ling FW, Buster JE. Emergency department diagnosis of ectopic pregnancy. Ann Emerg Med. 1990. 19:1098–1103.

13. Barnhart KT, Simhan H, Kamelle SA. Diagnostic accuracy of ultrasound above and below the β-hCG discriminatory zone. Obstet Gynecol. 1999. 94:583–587.
14. Garcia CR, Barnhart KT. Diagnosing ectopic pregnancy: Decision analysis comparing six strategies. Obstet Gynecol. 2001. 97:464–470.
15. Birken S, Kovalevskaya G, O'Connor J. Immunochemical measurement of early pregnancy isoforms of HCG: potential applications to fertility research, prenatal diagnosis, and cancer. Arch Med Res. 2001. 32:635–643.
16. Fein HG, Rosen RW, Weintraub BD. Increased glycosylation of serum human chorionic gonadotropin and subunits from eutopic and ectopic sources; comparison with placental and urinary forms. J Clin Endocrinol Metab. 1980. 50:1111–1120.

17. Wide L, Hobson B. Some qualitative differences of hCG in serum from early and late pregnancies and trophoblastic diseases. Acta Endocrinol (Copenh). 1987. 116:465–472.

18. Wide L, Lee JY, Rasmussen C. A change in the isoforms of human chorionic gonadotropin occurs around the 13th weeks of gestation. J Clin Endocrinol Metab. 1994. 78:1419–1423.
19. Norman RJ, Buck RH, Kemp MA, Joubert SM. Impaired corpus luteum function in ectopic pregnancy cannot be explained by altered human chorionic gonadotropin. J Clin Endocrinol Metab. 1988. 66:1166–1170.

20. Johnson MR, Riddle AF, Irvine R, Sharma V, Collins WP, Nicolaides KH, Grudzinskas JG. Corpus luteum failure in ectopic pregnancy. Hum Reprod. 1993. 8:1491–1495.
21. Lower AM, Yovich JL, Hancock C, Grudzinskas JG. Is luteal function maintained by factors other than chorionic gonadotropin in early pregnancy? Hum Reprod. 1993. 8:645–648.
22. Kratzer PG, Taylor RN. Corpus luteum function in early pregnancies is primarily determined by the rate of change of human chorionic gonadotropin levels. Am J Obstet Gynecol. 1990. 163:1497–1502.

23. Johnson MR, Riddle AF, Sharma V, Collins WP, Nicolaides KH, Grudzinskas JG. Placental and ovarian hormones in an embryonic pregnancy. Hum Reprod. 1993. 8:112–115.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download