Abstract
It has been suggested that dendritic cells (DCs) are critical antigen presenting cells for eosinophilic airway inflammation in a mouse model of asthma, and cysteinyl leukotrienes may play a role in DC trafficking in asthmatics. We investigated whether the number of DCs is increased in the induced sputum of both atopic and nonatopic asthmatics and is related to activated eosinophil count in the sputum. Sputum was induced by inhalation of hypertonic saline in 9 atopic and 12 nonatopic asthmatics and 10 nonatopic normal controls, and differential cell counts were performed. DCs and activated eosinophils were identified by immunocytochemistry with monoclonal antibodies (anti-CD1a and EG2, respectively). There were significantly higher percentages of eosinophils, EG2+ cells, and CD1a+ DC in the sputum of atopic and nonatopic asthmatics compared with normal controls, respectively. In asthmatics, the percentage of CD1a+ DC was significantly correlated with that of EG2+ cells (Rs=0.62, p=0.004). We demonstrated that the increased number of DCs was evident in the induced sputum of both atopic and nonatopic asthmatics, and the DC number was related to the activated eosinophil count, which suggests that DCs may contribute to the ongoing eosinophilic inflammation in asthmatic airways, and vice versa.
Asthma is a chronic inflammatory disorder of the airways (1), in which a CD4+ Th2 lymphocyte cytokine profile is instrumental in initiating and sustaining the inflammatory process (2). The CD4+ T cells need to be activated by antigen presenting cells (APCs). Dendritic cells (DCs) are the major APCs responsible for the activation of naïve T cells and the generation of primary T cell responses (3) and can also selectively activate Th2-like lymphocytes in asthma (4, 5). Therefore, DCs are most likely to contribute to the eosinophilic airway inflammation in asthma.
It has been demonstrated that DCs are critical APCs for the eosinophilic airway inflammation in sensitized mice (6). Upregulation of the DC network has also been suggested to be an integral part of the eosinophilic airway inflammation in sensitized rats (7). Recently, a study has demonstrated a role for cysteinly leukotrienes (CysLTs) in the migration of DCs from blood to the airway in asthmatics (8). CysLTs are produced primarily by mast cells, basophils, and eosinophils (9). It is reasonable to consider that DCs are closely related to the ongoing eosinophilic airway inflammation in asthma.
Bronchial biopsies have revealed the increased numbers of DCs in the airways of atopic (5, 10-14) and nonatopic asthmatics (13, 14). However, sputum induction has emerged as a useful non-invasive technique to assess the airway inflammation in subjects with asthma and has been shown to return reproducible data with regard to cellular and soluble markers of inflammation (15, 16). We reasoned that the increased number of DCs observed in bronchial biopsies could be detected in the induced sputum as well. Since it has been known that the degree of eosinophil activation is more important than the increase in number of eosinophils in reflecting the ongoing inflammation in asthma (17-19), it is expected that there is the relationship between DCs and activated eosinophils in asthmatic airways.
The aims of the present study are to determine whether the number of DCs is increased in the induced sputum of both atopic and nonatopic asthmatics, and to determine whether the number of DCs is closely related to the activated eosinophil count in the induced sputum.
Nine atopic and 12 nonatopic asthmatics and 10 healthy volunteers were included in the study. Asthma was diagnosed on the basis of clinical history of recurrent episodes of wheeze, breathlessness and/or cough associated with the demonstration of reversible airway obstruction or bronchial hyperresponsiveness to methacholine. Subjects were considered to have the significant reversibility if there was an improvement in FEV1 of >15% and 200 mL after salbutamol 200 µg. The methacholine bronchial provocation test was done according to the method of Chai et al. (20). The bronchial hyperresponsiveness was defined if the provocative concentration of methacholine that caused a 20% fall in FEV1 was <25 mg/mL (21). Atopy was defined as a positive skin prick test (mean wheal diameter ≥3 mm) to at least one of the common aeroallergens.
All asthmatics had a clinical history suggestive of asthma for at least 3 months before the study. Asthma symptoms were controlled with β2-adrenergic drugs on a continuous basis or on demand. None had received oral or inhaled corticosteroids for at least 6 weeks before the study. Normal controls were not taking any form of medication, had no history of asthma or other allergic diseases, and had no skin reactions to the common allergens.
All subjects had no upper respiratory tract infection within the preceding 4 weeks. Nonsmoking was not a prerequisite for selection. All subjects gave written informed consent for this study, which was approved by the Chonnam University Hospital Ethics Committee.
Sputum induction was performed by inhalation of hypertonic saline (NaCl 4.5%) 15 min after premedication with 200 µg of inhaled salbutamol. Aerosols were generated by an ultrasonic nebulizer (Devilbiss, Somerset, PA, U.S.A.), the output of which was set at 2.5 mL/min. The subjects were asked to inhale the aerosols for 30 min in total. Subjects discarded saliva into a bowl and mouth washed before each expectoration. Secretions collected during the first 5 min were discarded to minimize squamous cell contamination. Subjects were encouraged to cough deeply at 5 min intervals and any other time they felt the need. Samples were collected in a Petri dish. Sputum plugs of 1 mL were selected and kept at 4℃ until processing.
The sputum was treated with 4 times their volume of 0.1% dithiothreitol (DTT) (Sigma, St. Louis, MO, U.S.A.) at 37℃ for 15 min. This suspension was further diluted and mixed with phosphate buffered saline (PBS) in a volume equal to the sputum plus DTT for an additional 5 min. Cells were centrifuged at 1,000 rpm for 10 min and then re-suspended in PBS. Cyto-spins (CF-120, Tokyo, Japan) were prepared on glass slides and the preparations were stained with Diff-Quik (American Scientific Products, McGaw, IL, U.S.A.). All cells were enumerated and counted, which allowed calculation of both the squamous cell percentage of the total cell sample and the epithelial, macrophage, neutrophil, eosinophil, and lymphocyte percentages of the nonsquamous cells. At least 200 nonsquamous cells on each slide were read by the same investigator. Cytospins were also prepared on ProbeOn Plus microscope slides (Fisher Scientific, Pittsburgh, PA, U.S.A.) for immunocytochemical staining, and the preparations were fixed in methanol (Merck, Darmstadt, Germany) at 4℃ for 5 min and stored at -70℃ until use.
Immunostaining was performed using the following mouse IgG1 monoclonal antibodies: anti-CD1a (Clone 010; Dako, Carpinteria, CA, U.S.A.), directed against the CD1a antigen of DC (22), and EG2 (Kabi Pharmacia, Uppsala, Sweden), directed against the activated form of human eosinophil cationic protein.
The staining was detected by the LSAB®2 system (Dako). Sputum slides were pretreated with a solution of hydrogen peroxide and 15 mM sodium azide (Peroxidase blocking reagent; Dako) to inhibit endogenous peroxidase activity. After washes in Tris-buffered saline (TBS), a blocking solution containing 0.25% casein (Protein block serum-free; Dako) was applied to inhibit nonspecific staining. The slides were incubated with the monoclonal antibodies in the optimal dilution at room temperature for 30 min.
The monoclonal antibodies were used at working concentrations which were preliminarily determined (30 µg/mL for anti-CD1a and 1 µg/mL for EG2). After washes in TBS, the binding antibodies were labeled with biotinylated secondary antibody (Dako) during 10 min incubation and made visible using a streptavidin-horseradish-peroxidase complex detection system (Dako). Aminoethylcarbazole was applied as chromogen, resulting in a red reaction product. Finally, slides were rinsed with distilled water, counterstained with hematoxylin solution (Accustain; Sigma), and mounted in glycerin gelatin. Control slides were similarly treated either with the primary antibody omitted or replaced by an unrelated mouse IgG1 monoclonal antibody (Dako). The percentage of CD1a+ DC or EG2+ cells was determined from a count of 1,500 or 500 nonsquamous cells under light microscopy, respectively.
Results were expressed as median (range). Differences between groups were analyzed by the Mann-Whitney U test for continuous variables and 2 tests for dichotomous variables. The correlation between variables was examined by Spearman's rank correlation coefficient. A value of p<0.05 was considered significant.
The ages of atopic asthmatics did not differ from those of normal controls, but those of nonatopic asthmatics were significantly higher compared with normal controls (p<0.001). There were more females in atopic (p<0.05) or nonatopic asthmatics (p<0.05) than in normal controls, respectively. Five current smokers were included in the study. The percentage of current smokers did not significantly differ between atopic or nonatopic asthmatics and normal controls, respectively. Nonatopic asthmatics had four ex-smokers who quitted smoking 4.5 (2-15) yr before (Table 1).
There were no significant differences in sex, smoking habits, and baseline FEV1 between atopic and nonatopic asthmatics, except that the ages of atopic asthmatics were significantly lower than those of nonatopic asthmatics (p<0.01). Methacholine bronchial provocation tests were performed in eight atopic and six nonatopic asthmatics. The median values of PC20 were similar between atopic and nonatopic asthmatics (Table 1). Sputum induction was performed successfully and was well tolerated in all subjects. A nonatopic asthmatic patient with low baseline FEV1 of 1.2 L (38.9% of predicted) had a postbronchodilator FEV1 of 1.52 L (55%) before sputum induction.
The median percentage of squamous cells in all subjects was 6.1% (0-37%). The median percentage of macrophages was significantly lower in atopic (p<0.05) or nonatopic asthmatics (p<0.05) than in normal controls, respectively. The median percentage of eosinophils was significantly higher in atopic (p<0.001) or nonatopic asthmatics (p<0.001) than in normal controls, respectively. There were no significant differences in the median percentages of lymphocytes, neutrophils, and epithelial cells between atopic (p>0.05, respectively) or nonatopic asthmatics (p>0.05, respectively) and normal controls (Table 2).
CD1a+ DC (Fig. 1A) and EG2+ cells (Fig. 1B) were evident in the induced sputum. No immunostaining was identified either in the absence of primary antibody or in the presence of the mouse IgG1 isotype control. In one nonatopic asthmatic patient, EG2 immunostaining was not done because of a paucity of sputum cells. The median percentage of CD1a+ DC was significantly higher in atopic (0.27% [0.13-0.93%], p<0.01) or nonatopic asthmatics (0.17% [0-1.4%], p<0.05) than in normal controls (0.07% [0-0.2%]), respectively (Fig. 2A). The median percentage of EG2+ cells was significantly higher in atopic (0.6% [0-5.6%], p<0.01) or nonatopic asthmatics (0.8% [0-3%], p<0.01) than in normal controls (0% [0-0.2%]), respectively (Fig. 2B).
There were no significant differences in the percentages of sputum macrophages, lymphocytes, neutrophils, eosinophils, epithelial cells, CD1a+ DC, and EG2+ cells between atopic and nonatopic asthmatics (p>0.05, respectively) (Table 2 and Fig. 2).
When the analyses were performed according to sex or smoking habits, the percentage of CD1a+ DC did not differ between male and female asthmatics (0.23% [0-1.40%] vs. 0.27% [0-1.07%], p>0.05). In all asthmatics, the percentage of CD1a+ DC tended to be lower in four current smokers compared with the rest (0.07% [0-0.53%] vs. 0.27% [0.07-1.4%], p=0.08)
The percentage of CD1a+ DC did not correlate with that of macrophages (Rs=0.07, p>0.05), epithelial cells (Rs=-0.21, p>0.05), neutrophils (Rs=0.06, p>0.05), eosinophils (Rs=-0.14, p>0.05), or lymphocytes (Rs=0.36, p>0.05) in all asthmatics, respectively. However, there was a significant correlation between the percentages of CD1a+ DC and EG2+ cells in all asthmatics (Rs=0.62, p=0.004, n=20). When the analyses were limited to atopic or nonatopic asthmatics, the percentage of CD1a+ DC was correlated with that of EG2+ cells in atopic (Rs=0.65, p=0.06, n=9) or nonatopic asthmatics (Rs=0.64, p=0.03, n=11), respectively (Fig. 3). Additionally, the percentage of CD1a+ DC did not correlate with the ages in all asthmatics (Rs=-0.12, p>0.05).
We demonstrated that the number of DCs was significantly increased in the induced sputum of both atopic and nonatopic asthmatics compared with normal controls, and there was the close relationship between DCs and activated eosinophils in asthmatics. Sputum induction by inhalation of hypertonic saline solution has been validated and is now widely used to study airway secretions in patients with airway diseases such as asthma. This method, which is inexpensive and does not require complex instrumentation, has the advantages of being less invasive than bronchoscopy and bronchoalveolar lavage. Its safety has been studied in patients with asthma and found to be good even in patients with more severe disease (23, 24). The reproducibility of the method has been extensively assessed and found to be good with respect to cell counts and a number of soluble markers of inflammation (15, 16). Therefore, the first finding of showing the presence of DCs in the induced sputum can prompt future studies on DCs using the sputum in asthmatics.
The increased numbers of DCs in the airways of atopic asthmatics are compatible with their role of professional APCs involved in the allergen dependent activation of T lymphocytes in the lower respiratory tract of asthmatics (3). However, a few studies have shown the increased number of DCs in the bronchial mucosa of nonatopic asthmatics (13, 14). Our data extended their findings by showing that the increased number of DCs was evident in the induced sputum of nonatopic asthmatics, and the number of DCs was similar between atopic and nonatopic asthmatics. These observations suggest that DCs may play a role in the pathogenesis of nonatopic asthma as well as atopic asthma, although further investigations are needed.
It has been demonstrated that DCs are essential for the acquisition of effector function in memory Th2 cells and the subsequent development of eosinophilic airway inflammation in a mouse model of asthma (6), which may explain in part our finding of the relationship between DCs and activated eosinophils in the airways of atopic asthmatics. Lambrecht et al. (7). have demonstrated that the kinetics of increase of DCs after repeated exposure to antigen closely resemble those of Th2 cells and eosinophils in bronchoalveolar lavage fluid of previously sensitized rats, suggesting that these cells are attracted into the airways by similar mechanisms. Allergic airway inflammation is indeed accompanied by the local release of chemokines and mediators such as eotaxin, regulated on activation, normal T cells expressed and secreted, macrophage inflammatory protein-1α, monocyte chemotactic protein (MCP)-1, MCP-3, which can attract DCs (25, 26) as well as eosinophils. Schon-Hegrad et al. (27) reported that chronic eosinophilic airway inflammation in rats was accompanied by an increase of the intramucosal DCs network at areas of eosinophilic infiltration. In addition, DCs may produce chemokines that preferentially attract recently activated Th2 cells (28, 29), indicating that they might contribute directly to the ongoing eosinophilic inflammation. Recently, Parameswaran et al. (8) have demonstrated that CysLTs play a role in the migration of DCs from blood to the airway in asthma. CysLTs released from activated eosinophils (9) may contribute to DC trafficking, which may explain in part our finding that the DC number was correlated with only the number of activated eosinophils, but not the differential count of eosinophils. Collectively, it is probable that DCs may contribute to the ongoing eosinophilic inflammation in asthmatic airways, and vice versa. The hypothesis might explain why there is the relationship between DCs and activated eosinophils in the airways of nonatopic as well as atopic asthmatics.
Like other previous studies (5, 10-12, 14), we used a monoclonal antibody directed against CD1a antigen to identify airway DCs. Other cells known to express this marker are either present in the airways at very low frequency (B-lymphocytes) (30) or absent altogether (cortical thymocytes).
In the present study, there were significant differences in age and sex among the three groups, which might affect our results. However, this may be not the case, based on our findings that the number of DCs did not differ according to age or sex in all asthmatics. The present study included five current smokers in total, although there was no significant difference in the number among the three groups. Cigarette smoking might contribute to the increased number of DCs in the airways of asthmatics (31). However, the number of DCs was lower than expected in our current smokers, which could be explained by the small sample size of studied patients.
In conclusion, although our results referred to a limited number of asthmatics, we demonstrated that the increased number of DCs was evident in the induced sputum of both atopic and nonatopic asthmatics, and the DC number was related to the activated eosinophil count, which suggests that DCs may contribute to the ongoing eosinophilic inflammation in asthmatic airways, and vice versa. Further studies are needed to elucidate the precise mechanism for the relationship in asthma.
Figures and Tables
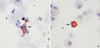 | Fig. 1Immunocytochemical staining with anti-CD1a (A) and EG2 (B) (×400). CD1a+ dendritic cell (arrow) and EG2+ cell (arrow) were
shown in the induced sputum of an asthmatic patient. |
 | Fig. 2CD1a+ dendritic cells (A) and EG2+ cells (B) in the induced sputum of atopic (n=9) and nonatopic (n=12 in panel A and n=11 in panel B) asthmatics and normal controls (n=10). Cell counts were expressed as percentage of total nonsquamous cells. Horizontal bars represent median values. |
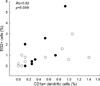 | Fig. 3Relationship between CD1a+ dendritic cells and EG2+ cells in the induced sputum of atopic (closed circle, n=9) and nonatopic (open circle, n=11) asthmatics. Cell counts were expressed as percentage of total nonsquamous cells. |
References
1. NIH Publication No 95-3659. NHLBI/WHO Workshop Report: Global strategy for asthma management and prevention. 1995. National Institutes of Health.
2. Ricci M, Rossi O, Bertoni M, Matucci A. The importance of Th2-like cells in the pathogenesis of airway allergic inflammation. Clin Exp Allergy. 1993. 23:360–369.

3. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.

4. Simon JC, Cruz PD Jr, Bergstresser PR, Tigelaar RE. Low dose ultraviolet B-irradiated Langerhans cells preferentially activate CD4+ cells of the T helper 2 subset. J Immunol. 1990. 145:2087–2091.
5. Bellini A, Vittori E, Marini M, Ackerman V, Mattoli S. Intraepithelial dendritic cells and selective activation of Th2-like lymphocytes in patients with atopic asthma. Chest. 1993. 103:997–1005.

6. Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998. 160:4090–4097.
7. Lambrecht BN, Carro-Muino I, Vermaelen K, Pauwels RA. Allergen-induced changes in bone-marrow progenitor and airway dendritic cells in sensitized rats. Am J Respir Cell Mol Biol. 1999. 20:1165–1174.

8. Parameswaran K, Liang H, Fanat A, Watson R, Snider DP, O'Byrne PM. Role for cysteinyl leukotrienes in allergen-induced change in circulating dendritic cell number in asthma. J Allergy Clin Immunol. 2004. 114:73–79.

10. Moller GM, Overbeek SE, Van Helden-Meeuwsen CG, Van Haarst JM, Prens EP, Mulder PG, Postma DS, Hoogsteden HC. Increased numbers of dendritic cells in the bronchial mucosa of atopic asthmatic patients: downregulation by inhaled corticosteroids. Clin Exp Allergy. 1996. 26:517–524.

11. Tunon-De-Lara JM, Redington AE, Bradding P, Church MK, Hartley JA, Semper AE, Holgate ST. Dendritic cells in normal and asthmatic airways: expression of the alpha subunit of the high affinity immunoglobulin E receptor (Fc epsilon RI-alpha). Clin Exp Allergy. 1996. 26:648–655.
12. Bertorelli G, Bocchino V, Zhou X, Zanini A, Bernini MV, Damia R, Di Comite V, Grima P, Olivieri D. Dendritic cell number is related to IL-4 expression in the airways of atopic asthmatic subjects. Allergy. 2000. 55:449–454.

13. Burke C, Power CK, Norris A, Condez A, Schmekel B, Poulter LW. Lung function and immunopathological changes after inhaled corticosteroid therapy in asthma. Eur Respir J. 1992. 5:73–79.
14. Bocchino V, Bertorelli G, Zhuo X, Grima P, Di Comite V, Damia R, Chetta A, Del Donno M, Foresi A, Casalini A, Testi R, Olivieri D. Short-term treatment with a low dose of inhaled fluticasone propionate decreases the number of CD1a+ dendritic cells in asthmatic airways. Pulm Pharmacol Ther. 1997. 10:253–259.

15. in't Veen JC, de Gouw HW, Smits HH, Sont JK, Hiemstra PS, Sterk PJ, Bel EH. Repeatability of cellular and soluble markers of inflammation in induced sputum from patients with asthma. Eur Respir J. 1996. 9:2441–2447.
16. Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, Gleich GJ, Dolovich J, Hargreave FE. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996. 154:308–317.

17. Adelroth E, Rosenhall L, Johansson SA, Linden M, Venge P. Inflammatory cells and eosinophilic activity in asthmatics investigated by bronchoalveolar lavage. The effects of antiasthmatic treatment with budesonide or terbutaline. Am Rev Respir Dis. 1990. 142:91–99.

18. Ronchi MC, Piragino C, Rosi E, Stendardi L, Tanini A, Galli G, Duranti R, Scano G. Do sputum eosinophils and ECP relate to the severity of asthma? Eur Respir J. 1997. 10:1809–1813.

19. Virchow JC Jr, Holscher U, Virchow C Sr. Sputum ECP levels correlate with parameters of airflow obstruction. Am Rev Respir Dis. 1992. 146:604–606.

20. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975. 56:323–327.

21. Kim SH, Oh SY, Oh HB, Kim YK, Cho SH, Kim YY, Min KU. Association of beta2-adrenoreceptor polymorphisms with nocturnal cough among atopic subjects but not with atopy and nonspecific bronchial hyperresponsiveness. J Allergy Clin Immunol. 2002. 109:630–635.
22. Murphy GF, Bhan AK, Sato S, Mihm MC Jr, Harrist TJ. A new immunologic marker for human Langerhans cells. N Engl J Med. 1981. 304:791–792.

23. Wong HH, Fahy JV. Safety of one method of sputum induction in asthmatic subjects. Am J Respir Crit Care Med. 1997. 156:299–303.

24. de la Fuente PT, Romagnoli M, Godard P, Bousquet J, Chanez P. Safety of inducing sputum in patients with asthma of varying severity. Am J Respir Crit Care Med. 1998. 157:1127–1130.

25. Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995. 155:3292–3295.
26. McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, Stumbles PA, Wells TN, Holt PG. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996. 184:2429–2432.

27. Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991. 173:1345–1356.

28. Hashimoto S, Suzuki T, Dong HY, Nagai S, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocyte-derived dendritic cells. Blood. 1999. 94:845–852.

29. Lieberam I, Forster I. The murine beta-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur J Immunol. 1999. 29:2684–2694.
30. Poston RN, Chanez P, Lacoste JY, Litchfield T, Lee TH, Bousquet J. Immunohistochemical characterization of the cellular infiltration in asthmatic bronchi. Am Rev Respir Dis. 1992. 145:918–921.

31. Soler P, Moreau A, Basset F, Hance AJ. Cigarette smoking-induced changes in the number and differentiated state of pulmonary dendritic cells/Langerhans cells. Am Rev Respir Dis. 1989. 139:1112–1117.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download