Abstract
This study is the first report of genetic and environmental influences on birthweight using Korean twins. The sample consisted of 255 monozygotic (MZ) and 178 dizygotic (DZ) twin pairs drawn from the Seoul Twin Family Study. Intraclass twin correlations were computed for the twins' birthweights obtained from parents (typically mothers) of the twins. To estimate genetic and shared and nonshared environmental influences on birthweight, standard univariate model-fitting analyses were performed using a software, Mx. For each gender, MZ twin correlations were higher than DZ twin correlations, suggesting existence of genetic influences on birthweight; however, DZ twin correlations were higher than half the MZ twin correlations, indicating that shared environmental factors are also important. For each zygosity, twin correlations were not significantly different between males and females, implicating that genes and environments that cause individual differences in birthweight may not vary between males and females. Model-fitting analyses based on the data pooled across gender yielded estimates of 17% for genetic, 60% for shared environmental, and 23% for nonshared environmental influences on birthweight.
Birthweight is an important predictor of mental and physical health in later life. Birthweight has been shown to be associated with height and body mass index (1), susceptibilities to attention deficit hyperactivity disorder and depressive symptoms in adolescence (2), and the risk factors for schizophrenia (3), diabetes, hypertension, cardiovascular disease, and respiratory illnesses in adulthood (4, 5). Birthweight also has implications for later behavioral characteristics; Low birthweight has shown to be related to later poor social adjustment (6), psychosocial distress (7), academic achievement (8), and IQ (9, 10).
Birthweight is a function of the fetal genotype and the intrauterine environment. Research with monozygotic (MZ) and dizygotic (DZ) twins provides a unique opportunity to distinguish between genetic and environmental causes of phenotypic variations in human populations. Specifically, the classical twin method decomposes the total phenotypic variance into variance components attributable to genetic, shared environmental, and nonshared environmental factors, respectively (11-14). MZ twins share identical genes, whereas DZ twins share on average 50% of their segregating genes. For this reason, genetic effects are indicated if MZ twins are more similar than DZ twins. Shared environmental factors correspond to those environmental factors that are shared by the members of a twin pair. Because shared environmental factors operate to make twin pairs alike, the importance of shared environmental effects is indicated if the similarity for DZ twins who only share 50% of genes is greater than half the similarity for MZ twins who share 100% of their segregating genes. For birthweight, shared environment refers to the characteristics of the fetal uterine environment that are constant across the members of a twin pair. Thus, maternal substance use and abuse, smoking, and uterine size can act as shared environmental factors in twin studies of birthweight.
The term, nonshared environmental factors refer to those environmental factors that are not shared by the members of a twin pair. Because nonshared environmental effects always operate to make twins dissimilar, the effects are manifested as within-pair differences. Examples of nonshared environmental factors that can lead to within-pair differences in birthweight include unequal partitioning of nutrients and blood supply between the twins in utero (15, 16). Measurement errors will be confounded in the estimate of nonshared environmental effects.
Previous twin studies of birthweight have shown moderate heritability and substantial shared environmental influences (17-19). Typically, heritability estimates were between 20% and 40% and the shared environmental estimate was approximately 55%. Similar estimates were found in a study where preterm twins were analyzed (20), suggesting that the same group of genes might operate in variation in birthweight of the extreme group.
Most twin studies of birthweight so far have been conducted on the basis of Western populations. Because heritability estimates are population-specific, populations with various genetic affinities, social environments, health care systems, and dietary habits can yield different estimates of genetic and environmental influences. Until now, no twin studies examined genetic and environmental effects on variations in birthweight in the Korean population. The present study investigates these effects using a sample of Korean twins.
The present sample comprised MZ and DZ twins drawn from the Seoul Twin Family Study (STFS). The STFS is a large, longitudinal twin family study of genetic and environmental influences on the behavioral development of children and adolescents in Seoul, Korea. The STFS registry contains names, mailing addresses, and telephone numbers of approximately 4,600 twin pairs ascertained from all private and public elementary, middle, and high schools in Seoul. The registry was constructed with the assistance of Seoul Metropolitan Office of Education in the years 2001 and 2002. Further details of the study design and recruitment procedures of the STFS are described in Hur (2002) (21).
As part of the STFS mail survey in the year 2002, a booklet that included questions on twins' birthweight, zygosity, and demographic information was mailed to the parents of the elementary school twin participants of the STFS. On the STFS register, there were 2,945 elementary school twin pairs. About 18 percent of the twins returned the booklet. However, given that some twins had moved away and that there were some incorrect mailing addresses, it is likely that the true response rate was higher than 18%. Of the twins who returned the booklet, cases were selected out for the present analyses because (a) parents reported that they did not remember one or both of their twin children's birthweight, (b) parents simply skipped questions on one or both of their twin children's birthweight, (c) parents did not complete the zygosity questionnaire explained below, and (d) twins' zygosity was ambiguous.
The final sample for the present analyses included 255 MZ and 178 DZ twin pairs. MZ twins of the present sample consisted of 123 male pairs (MZM) and 132 female pairs (MZF), whereas DZ twins included 44 male pairs (DZM) and 56 female pairs (DZF) of the same-sex twins and 78 pairs of the opposite-sex twins (DZOS). Ages of the twins at the time of the survey ranged from 7 to 14 yr, with a mean (SD) of 10.31 yr (1.57 yr) for MZM, 10.60 yr (1.75 yr) for MZF, 10.50 yr (1.75 yr) for DZM, 10.16 yr (1.56 yr) for DZF, and 10.45 yr (1.61 yr) for DZOS.
The mean ages of parental ages were 32.9 yr (SD=4.3 yr) for fathers and 30.0 yr (SD=4.0 yr) for mothers when twins were born. The average years of parental education were 13.8 yr for fathers and 12.8 yr for mothers. These figures were only slightly higher than the average years of education for males (12.6 yr) and females (11.5 yr) for the corresponding age group in Korea (22), indicating that parents of the twins in the present sample are fairly representative of Korean adults in terms of educational attainment.
Zygosity was determined from responses by parents (typically, mothers) of the twins to eight questions concerning the twins' physical similarity, the parents' beliefs concerning their twin children's zygosity, and frequencies of confusion by family members and others. Although this questionnaire method to determine zygosity has been widely used in large twin studies (1, 18, 19) and is regarded over 90% accurate for twins in general (23), as explained earlier, cases where zygosity could not be classified with certainty were excluded from data analyses.
Data analyses consisted of two parts: comparison of twin intraclass correlations and model-fitting analyses. Intraclass correlations were computed for the five twin groups (MZM, MZF, DZM, DZF, & DZOS) from mean squares between (MSB) and within (MSW) twin-pairs in a one-way ANOVA according to the standard formula: (MSB-MSW)/(MSB+MSW). For each sex, two comparisons were made: (a) MZ vs. same-sex DZ twins, and (b) same-sex vs. opposite-sex DZ twins. For each zygosity, male versus female twin correlations were compared.
Fig. 1 presents the path diagram for the univariate biometrical model for the classical twin design (11, 12, 24). In Fig. 1, squares represent observed variables (i.e., VP1=the phenotypic variance of birthweight for the first twins, VP2=the phenotypic variance of birthweight for the second twins), and circles represent latent variables that influence the phenotypic variances of birthweight for the first and the second twins. Thus, VA1, VC1, and VE1, respectively, represent genetic, shared environmental, and nonshared environmental variances that influence the phenotypic variance of birthweight for the first twins, and VA2, VC2, and VE2 denote the corresponding variances that influence the phenotypic variance of birthweight for the second twins.
According to the model in Fig. 1, the phenotypic variances of birthweight for the first and the second twin are linear functions of genetic, shared environmental, and nonshared environmental variances. That is,
VP1=VA1+VC1+VE1
VP2=VA2+VC2+VE2.
The correlations between VA1 and VA2 were set as 1.0 for MZ twins and 0.5 for DZ twins because MZ twins are genetically identical, whereas DZ twins share on average 50% of their segregating genes (14). Both for MZ and for DZ twins, the correlations between VC1 and VC2 were set as 1.0 because, by definition, shared environmental variance represent environmental factors shared by two members of a twin pair. VE1 and VE2 are not connected with correlation because they represent environmental factors that are not shared by two members of a twin pair. Since we do not expect the magnitudes of genetic, shared environmental, and nonshared environmental variances to be different between the first and the second twins (i.e., VP1=VP2, VC1=VC2, and VE1=VE2), the expected variances and covariances of birthweight for the MZ and for the DZ twins can be presented by the following three equations:
VP=VA+VC+VE
MZCOV=VA+VC
DZCOV=0.5 VA+VC.
Using a maximum-likelihood estimation procedure in Mx (24), the observed variances and covariances of birthweight for the MZ and for the DZ twins were fitted to the expected variances and covariances explained above. By minimizing the discrepancy between the observed and the expected variances and covariances of birthweight for the MZ and for the DZ twins, Mx estimates genetic (VA), shared (VC), and nonshared environmental variance (VE) parameters and yields a goodness of fit index that is distributed as a χ2.
In order to assess significance of the genetic and the shared environmental variance parameters, χ2s from the reduced models that eliminated (a) the genetic parameter (Model 1 in Table 2) and (b) the shared environment parameter (Model 2 in Table 2), were compared with χ2 from the full model that contained all three of the genetic, shared environmental, and nonshared environmental variance parameters. If the models are nested, the change in χ2 is itself distributed as a χ2, which permits evaluation of the significance of the eliminated parameters (25). A nonsignificant change in χ2 in the reduced model as contrasted with the full model indicates that the reduction of the parameters does not significantly worsen the fit of the model to the data, whereas a significant change in χ2 indicates that the parameters should be retained in the full model to adequately explain the data.
Birthweights were not significantly different between MZ and DZ twins for each sex. First-born twins weighed slightly but significantly more than second-born twins (average birthweight: 2.50 kg vs. 2.43 kg; t=2.03, p<0.05). Also, male twins weighed significantly more than female twins (average birthweight: 2.55 kg vs. 2.42 kg; t=3.94, p<0.001).
Table 1 presents means, standard deviations, and twin intraclass correlations for birthweight for the five twin groups (MZM, MZF, DZM, DZF, & DZOS). The hypothesis that twin correlations were homogeneous across gender (male vs. female) or zygosity (MZ vs. DZ) was tested using the Fisher's z transformation method (26). Correlations were not significantly different between males and females in any of the zygosity group. This finding of no significant gender differences in twin correlations for birthweight was consistent with the results reported in a sample of Belgium twins (27). For each gender, the MZ correlation was higher than the DZ correlation, indicating the existence of genetic component in birthweight. However, the DZ correlation was higher than half the MZ correlation, suggesting that shared environmental influences are substantial in birthweight. The opposite-sex twin correlation was not significantly different from the same-sex twin correlation, implying that genes and environments that cause individual differences in birthweight may not vary in males and females.
Because we did not observe any significant differences in comparing male and female correlations, model-fitting analyses were performed on the basis of the combined sample of males and females. Prior to model-fitting analyses, birthweight was corrected for gender to remove the mean effect of gender using regression analyses.
Table 2 presents the model-fitting results. The full model provided a good fit to the data, yielding a nonsignificant χ2 value of 1.39 for 3 df (p=.71). When the genetic parameter (VA) was eliminated from the full model (Model 1 in Table 2), a significant change in χ2 was observed, suggesting that genetic factor is important for birthweight. A reduction of the shared environment parameter (VC) from the full model also yielded a significant difference in χ2 (Model 2 in Table 2). These results suggest that the full model with genetic, shared environment, and nonshared environment parameters appropriately explain variation in birthweight. Genetic and shared and nonshared environmental influences on birthweight estimated from the full model were, respectively, 17%, 60%, and 23%. These estimates generally conformed to the impression gained from an examination of the twin intraclass correlations.i
Knowledge on the importance of genetic and environmental factors in birthweight has implications for understanding the physiology of pregnancy. So far, the relative influence of genetic and environmental contributions to individual difference in birthweight has rarely been examined in the Korean population. The present study indicated that variation in birthweight in Koreans were largely explained by the shared environment of the fetuses, not much by fetal genes or by environmental factors unique to each fetus. The variance of birthweight attributable to genetic factors was 17%, whereas variances due to shared and nonshared environmental factors were 60% and 23%, respectively. The confidence intervals of these estimates (see Table 2) agree with those typically obtained from Western twin samples.
What are the variables that explain shared environmental components in birthweight? In the present study, due to a lack of data, the effects of gestational age were not estimated separately. However, it is likely that gestational age at delivery contributed to the estimate of shared environmental influence in the present analysis, because gestational age is the same for the members of a twin pair for both MZ and DZ twins. Indeed, a previous twin study (27) has shown that gestational age explained the largest proportion of shared environmental variance in birthweight.
The findings of large shared environmental effects in birthweight in the present study also imply that, consistent with prior studies (28-30), maternal smoking, substance use, nutrition, obstetrical care, and socioeconomic status that influence the uterine environment are important in determining birthweight. It should, however, be noted that the characteristics of the uterine environment of the fetus depend not only by factors such as maternal smoking and SES, but also by maternal physical or physiological characteristics that are presumably under the control of maternal genes. For example, a few investigators found associations between birthweight and height and weight of mothers (1). There were very modest, but statistically significant correlations between birthweight and maternal and paternal heights (r=.09 for maternal; r=.10 for paternal) in the present sample also. Maternal genes influencing birthweight may also operate through susceptibility to diseases during pregnancy. For example, genetic factors have been shown to influence risk of pre-eclampsia and gestational hypertension (31). As is well known, pre-eclampsia and gestational hypertension are important risk factors for the fetal growth retardation (32).
At present little is known about maternal or fetal genes that are involved in birthweight. Recently, Hocher et al. (33) reported that the maternal C825T allele of the GNB3 gene is involved in birthweight. Unfortunately, however, subsequent study by Masuda et al. (34) could not replicate the association of the maternal C825T allele with birthweight. Busch et al. (35) studied neonates of South Asian ancestry and found that variation in PON2 genes were associated with birthweight: Significantly lower birthweights were observed among those who were homozygous for PON2 A148/A148, as compared with those with the other two genotypes. Replication studies, however, will be necessary to strengthen these findings.
The results of the present study indicate that about 23% of the variation in birthweight was attributable to nonshared environmental influences including measurement error. As explained earlier, nonshared environmental influences are manifested as within-pair differences. Studies of MZ twins who are discordant for birthweight provide an excellent opportunity to test whether intrauterine programming has an enduring impact on health outcome later in life. If difference in birthweight within MZ twin pairs is correlated with later health outcome, this association cannot have a genetic basis and must be due to environmental experiences in utero that are not shared by the members of a twin pair. Studies that correlated within-pair differences in height and weight in adult twins with within-pair differences in birthweight found consistent evidence for the importance of the intrauterine environment in determining later height and weight. For example, Allison et al. (36) found in a large scale twin study that the correlation of within-pair difference in birth weight with within-pair difference in adult height was 0.32 (p<0.01), and with adult weight 0.14 (p<0.01), suggesting that the intrauterine environmental factors exert a long lasting influence on adult height and weight. Studies of within-pair difference in birthweight have also looked at later blood pressure (37, 38), non-insulin dependent diabetes (39), and acute myocardial infarction (40). The results of these studies, however, were inconsistent, pointing to a need for further studies with large samples.
A limitation of the present study is that birthweights were reported by the twins' mothers retrospectively. Maternal report of birthweight has been widely used for epidemiological studies (e.g., 1, 19). Validation studies have reported that the maternal recall of birthweight reasonably agrees with those on the obstetric records (41-43). For example, McCormick and Brooks-Gunn (42) compared the maternal recall of birthweight for 1,833 children of 8 to 10 yr of age with data from the hospital record. The correlation of birthweights reported by mothers 8 to 10 yr later with those on the medical records was .89.
Although it was not possible to verify the birthweight data in the present study by the medical record, the mean (SD) birthweight in the present analyses (2.49±0.50 kg, see Table 1) was fairly close to the national average of birthweight for twins found in the data from Korean birth certificate in 1996 (2.57±0.58 kg) (44) and slightly higher than the means of birthweight (typically, 2.15 to 2.43 kg) for Korean twins reported from the hospital records (45-50). In general, the average birthweights found in population-based studies tend to be higher than those obtained from the hospital records, because hospital records usually include high rates of high risk pregnancy as well as stillborn cases (44).
If mothers of MZ twins tend to recall their twins' birthweights as more similar than they are in reality, then this recall bias would lead to an inflation of the estimate of genetic effects. The estimate of genetic influence found in the present study, however, was not unusually high as compared to those reported in previous studies on the basis of Caucasian twin samples. Taken together, these results suggest that mothers' reports in the present study may be reasonably accurate, and even if there was inaccuracy in reporting, the inaccuracy is unlikely to have seriously affected the estimates of genetic and environmental influences on birthweight. Replication studies using the medical records will be needed to confirm the present findings, however.
Figures and Tables
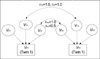 | Fig. 1Standard univariate model for birthweight. VP1, the phenotypic variance of birthweight for the first twins; VP2, the phenotypic variance of birthweight for the second twins; VA1, genetic variance for the first twins; VA2, genetic variance for the second twins; Vc1, shared environmental variance for the first twins; Vc2, shared environmental variance for the second twins; VE1, nonshared environmental variance for the first twins; VE2, nonshared environmental variance for the second twins. MZ, monozygotic twins; DZ, dizygotic twins. |
ACKNOWLEDGEMENTS
The thanks author twins and their parents who participated in the Seoul Twin Family Study.
References
1. Pietilainen KH, Kaprio J, Rasanen M, Winter T, Rissanen A, Rose RJ. Tracking of body size from birth to late adolescence: Contributions of birth length, birthweight, duration of gestation, parent's body size, and twinship. Am J Epidemiol. 2001. 154:21–29.
2. Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997. 38:931–941.

3. Kunugi H, Urushibara T, Murray RM, Nanko S, Hirose T. Prenatal underdevelopment and schizophrenia: a case report of monozygotic twins. Psychiatry Clin Neurosci. 2003. 57:271–274.

4. Eriksson M, Wallander MA, Krakau I, Wedel H, Svardsudd K. Birthweight and cardiovascular risk factors in a cohort followed until 80 years of age: the study of men born in 1913. J Intern Med. 2004. 255:236–246.
5. Shaheen SO, Sterne JA, Tucker JS, Florey CD. Birthweight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998. 53:549–553.
6. Brooks-Gunn J, Klebanov PK, Liaw F, Spiker D. Enhancing the development of low-birthweight, premature infants: changes in cognition and behavior over the first three years. Child Dev. 1993. 64:736–753.

7. Cheung YB, Ma S, Machin D, Karlberg J. Birthweight and psychological distress in adult twins: a longitudinal study. Acta Paediatr. 2004. 93:965–968.

8. Klein NK, Hack M, Breslau N. Children who were very low birthweight: development and academic achievement at nine years of age. J Dev Behav Pediatr. 1989. 10:32–37.
9. Boomsma DI, van Beijsterveldt CE, Rietveld MJ, Bartels M, van Baal GC. Genetics mediate relation of birthweight to childhood IQ [Letters]. BMJ. 2001. 323:1426–1427.
10. Luciano M, Wright MJ, Martin NG. Exploring the etiology of the association between birthweight and IQ in an adolescent twin sample. Twin Res. 2004. 7:62–71.

11. Neale MC, Cardon LR. Methodology for genetic studies of twins and families. 1992. London: Kluwer.
12. Heath AC, Neale MC, Hewitt JK, Eaves LJ, Fulker DW. Testing structural equation models for twin data using LISREL. Behav Genet. 1989. 19:9–35.

13. Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 2000. 4th ed. New York: Worth.
14. Falconer DS, MacKay TF. Introduction to quantitative genetics. 1996. 4th ed. Harlow, UK: Longman.
15. Machin G, Still K, Lalani T. Correlations of placental vascular anatomy and clinical outcomes in 69 monochorionic twin pregnancies. Am J Med Genet. 1996. 61:229–236.

16. Phillips DI, Davies MJ, Robinson JS. Fetal growth and the fetal origins hypothesis in twins-problems and perspectives. Twin Res. 2001. 4:327–331.
17. Nance WE, Kramer AA, Corey LA, Winter PM, Eaves LJ. A causal analysis of birthweight in the offspring of monozygotic twins. Am J Hum Genet. 1983. 35:1211–1223.
18. Whitfield JB, Treloar SA, Zhu G, Martin NG. Genetic and non-genetic factors affecting birthweight and adult body mass index. Twin Res. 2001. 4:365–370.

19. Johansson M, Rasmussen F. Birthweight and body mass index in young adulthood: the Swedish young male twins study. Twin Res. 2001. 4:400–405.

20. Clausson B, Lichtenstein PL, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000. 107:375–381.

21. Hur YM. Seoul Twin Family Study: design, sampling, assessments, and future directions. Twin Res. 2002. 5:389–393.

22. Korean National Statistical Office. Population & Housing Census Report. Social Indicators in Korea 2002. Seoul: Author.
23. Jackson RW, Snieder H, Davis H, Treiber FA. Determination of twin zygosity: a comparison of DNA with various questionnaire indices. Twin Res. 2001. 4:12–18.

24. Neale MC. Mx: Statistical modeling. 1999. 5th ed.Box 126 Medical College of Virginia, Richmond, VA 23298: Department of Psychiatry.
25. Hayduk LA. Structural equation modeling with LISREL: Essentials and Advances. 1988. Baltimore: Johns Hopkins.
26. Donner A, Rosner B. On inferences concerning a common correlation coefficient. Appl Stat. 1980. 29:69–76.

27. Vlietinck R, Derom R, Neale MC, Maes H, van Loon H, Derom C, Thiery M. Genetic and environmental variation in the birth weight of twins. Behav Genet. 1989. 19:151–161.

28. Luke B, Min SJ, Gillespie B, Avni M, Witter FR, Newman RB, Mauldin JG, Salman FA, O'Sullivan MJ. The importance of early weight gain in the intrauterine growth and birth weight of twins. Am J Obstet Gynecol. 1998. 179:1155–1161.

29. Hellerstedt WL, Himes JH, Story M, Alton IR, Edwards LE. The effects of cigarette smoking and gestational weight change on birth outcomes in obese and normal-weight women. Am J Public Health. 1997. 87:591–596.

30. Pattenden S, Dolk H, Vrijheid M. Inequalities in low birth weight: parental social class, area deprivation, and "lone mother" status. J Epidemiol Community Health. 1999. 53:355–358.

31. Sutherland A, Cooper DW, Howie PW, Liston WA, MacGillivray I. The incidence of severe pre-eclampsia amongst mothers and mothers-in-law of pre-eclamptics and controls. Br J Obstet Gynaecol. 1981. 88:785–791.

32. Tjoa ML, Oudejans CB, van Vugt JM, Blankenstein MA, van Wijk IJ. Markers for presymptomatic prediction of pre-eclampsia and intrauterine growth restriction. Hypertens Pregnancy. 2004. 23:171–189.

33. Hocher B, Slowinski T, Stolze T, Pleschka A, Neumayer HH, Halle H. Association of maternal G protein Beta3 subunit 825T allele with low birthweight. Lancet. 2000. 355:1241–1242.
34. Masuda K, Osada H, Iitsuka Y, Seki K, Sekiya S. Positive association of maternal G protein beta3 subunit 825T allele with reduced head circumference at birth. Pediatr Res. 2002. 52:687–691.

35. Busch CP, Ramdath DD, Ramsewak S, Hegele RA. Association of PON2 variation with birth weight in Trinidadian neonates of South Asian ancestry. Pharmacogenetics. 1999. 9:351–356.

36. Allison DB, Paultre F, Heymsfield SB, Pi-Sunyer FX. Is the intrauterine period really a critical period for the development of adiposity? Int J Obes Relat Metab Disord. 1995. 19:397–402.
37. Dwyer T, Blizzard L, Morley R, Ponsonby AL. Within pair association between birth weight and blood pressure at age 8 in twins from a cohort study. BMJ. 1999. 319:1325–1329.

38. Poulter NR, Chang CL, MacGregor AJ, Snieder H, Spector TD. Association between birth weight and adult blood pressure in twins: Historical cohort study. BMJ. 1999. 319:1330–1333.

39. Baird J, Osmond C, MacGregor A, Snieder H, Hales CN, Phillips DI. Testing the fetal origins hypothesis in twins: The Birmingham twin study. Diabetologia. 2001. 44:33–39.

40. Hubinette A, Cnattingius S, Ekbom A, de Faire U, Kramer M, Lichtenstein P. Birthweight, early enviornment, and genetics: a study of twins discordant for acute myocardial infarction. Lancet. 2001. 357:1997–2001.
41. Seidman DS, Slater PE, Ever-Hadani P, Gale R. Accuracy of mothers' recall of birthweight and gestational age. Br J Obstet Gynaecol. 1987. 94:731–735.

42. McCormick MC, Brooks-Gunn J. Concurrent child health status and maternal recall of events in infancy. Pediatrics. 1999. 104:1176–1181.
43. Pless CE, Pless IB. How well they remember. The accuracy of parent reports. Arch Pediatr Adolesc Med. 1995. 149:553–558.
44. Park SH, Lim KS, Ku SY, Kim SH. Study on multiple birth based on birth certificate data. Korean J Obstet Gynecol. 2000. 43:1253–1257.
45. Kim KS, Cha IA, Kim KB. A clinical study on twins. J Korean Pediatr Soc. 1994. 37:537–546.
46. Lim JE, Park SH, Cho KM, Sul HJ, Kim T, Kim HJ, Kang JS, Na JY. Perinatal outcomes according to intrapair birth weight difference in twin gestations. Korean J Obstet Gynecol. 2003. 46:509–513.
47. Oh KY, Park MH, Yang YS, Hwang IT, Park JS. The comparison of perinatal outcomes in twin and singleton pregnancies delivered prematurely between 28 weeks and 36 weeks gestational age. Korean J Obstet Gynecol. 2002. 45:1816–1820.
48. Lim JH, Kim HS, Hwang KJ, Yang JI, Kim MR, Lee HJ, Lim JC, Oh KS, Ryu HS. Twin pregnancies: In Vitro Fertilization vs. spontaneously conceived. Korean J Obstet Gynecol. 2002. 45:2172–2176.
49. Yang SH, Park SH, Choi SM, Seo YS, Roh CR, Chung JH. A comparison of perinatal outcomes according to the degrees of birth weight discordance in twin gestations. Korean J Obstet Gynecol. 1998. 41:2312–2317.
50. Kim KA, Min UG, Lim JW, Jun NL, Won HS, Kim CH, Kim EA, Lee PR, Lee IS, Kim KS, Kim A, Pi SY. Maternal and neonatal outcome of twin pregnancies after in vitro fertilization and embryo transfer. J Korean Pediatr Soc. 2003. 46:224–229.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download