Abstract
Most colonic multiple mucosa-associated lymphoid tissue (MALT) lymphomas are confirmed with a histologic and immunohistochemical staining of the mucosal biopsy specimen obtained during colonoscopic examinations. Endoscopically, colonic MALT lymphomas frequently appear as protruding and/or ulcerative lesions, and there are not so many reports of colonic MALT lymphoma as compared to the frequent reports of MALT lymphoma of stomach. We report a unique case of colonic MALT lymphoma presenting as a simple reddish discoloration of mucosa; this presentation has never been describe before. Our patient was a 47-yr-old male who suffered from tenesmus and mucoid stool. A colonoscopy was accomplished, followed by a histologic examination and we diagnosed a colonic MALT lymphoma. Staging of the disease was done because this was necessary for choosing the modality of treatments. The patient was then treated with polychemotherapy in conjunction with radiation therapy.
Isaacson and Wright first used the term mucosa-associated lymphoid tissue (MALT) lymphoma in 1983 and these MALT lymphomas are of interest as they arise from sites normally devoid of lymphoid tissue. They are frequently preceded by chronic inflammatory disorders (usually autoimmune in character) that result in the accumulation of lymphoid tissue (1).
The gastrointestinal tract is the most frequently involved extranodal location for MALT lymphomas and the stomach is the most common site for MALT lymphomas of the gastrointestinal tract. MALT lymphomas of the large intestine are very rarely observed.
Colonic MALT (mucosa-associated lymphoid tissue) lymphomas have not been well investigated when compared to stomach MALT lymphomas. There are various identifying features of colonic MALT lymphoma. Most of the colonic MALT lymphomas present as a protruding mass and/or an ulceration.
Here we report the first case of a mucosa-associated lymphoid tissue lymphoma manifesting itself colonoscopy as multiple mucosal discolorations alone and some localized granularity of the colonic mucosa in a 47-yr-old patient. Complete remission was achieved with anti-cancer polychemotherapy and radiation therapy and he has been free of disease for 14 months.
A 47-yr-old man suffering from tenesmus and mucoid stool was admitted to our hospital via a local clinic. He had smoked one pack of cigarettes daily for 20 yr and he did not drink alcohol. He had a history of pulmonary tuberculosis 20 yr ago that was treated with anti-tuberculosis medication for 9 months. On admission, he appeared in good health without any complaints of weight loss, fever and night sweats. His blood pressure was 110/90 mmHg, his pulse rate was 78/min, body temperature was 36.2℃ and respirations were 18/min.
On physical examination, the patient appeared normal and showed no remarkable findings except a depressed anterior chest wall. No lymphadenopathy was detected. His breath sounds were clear and his heart sounds were regular without any murmurs.
The laboratory findings included hemoglobin 13.4 g/dL, white blood cells 4,600/µL, platelets 305,000/µL, AST 22 IU/L, ALT 35 IU/L, ALP 47 IU/L, GGT 42 IU/L, LDH 393 U/L, BUN 14.8 mg/dL, creatinine 1.0 mg/dL, uric acid 6.2 mg/dL and calcium 3.6 mg/dL.
A radiograph of the chest showed a white lesion in the right upper lung field; this was thought to be a healed scar from pulmonary tuberculosis.
The upper gastrointestinal endoscopic examination revealed an healed ulcer in the duodenal bulb. Rapid urease test for Helicobacter pylori gave a negative result. Colonoscopic examination revealed two lesions 1) a granular & reddish mucosal thickening at the just above rectum (Fig. 1A), and 2) a reddish inflammatory mucosa without elevation or depression at the appendiceal orifice (Fig. 1B). Microscopic examination of the mucosal lesions of the colon and rectum showed lymphoepithelial lesions consisting of diffuse proliferation of small lymphocytes and some glandular destruction by lymphocytes (Fig. 2A). Immunohistochemistry showed the neoplastic cells were positive for CD20 (Fig. 2B) but negative for CD5, CD10, and cyclin D1. We then started to perform the staging evaluation of non-Hodgkin's lymphoma (NHL). The titer of β2-microglobulin was 1,770 µg/L and this was in the normal range. The proteins showed a pattern of polyclonal gammopathy upon serum protein electrophoresis. Computed tomography of abdomen revealed no lymphadenopathy. A bone marrow aspiration and biopsy was performed and they revealed no remarkable findings.
He was diagnosed with a low grade MALT lymphoma of stage IE (using Ann-Arbor staging) and stage II (using St. Jude Children's Research Hospital staging). He had received anticancer chemotherapy (cyclophosphamide 1,200 mg, adriamycin 80 mg, vincristine i.v. and prednisolone 100 mg p.o. for 5 days every month for 4 months) with involved field radiation therapy (3,000 cGy at the colon and 150 cGy at the rectum with 10 MV radiography 2 ports for 20 times during 29 days).
His presenting symptoms, including the tenesmus and mucoid stools subsided after treatment. Three months after treatment, a follow-up colonoscopy revealed no abnormal mucosa (Fig. 3).
Marginal zone B-cell lymphomas account for approximately 8% of all NHL, among which, they are the third most frequent histologic subtype (after the diffuse large B-cell lymphoma and the follicular lymphoma) (2). The group of lymphomas classified as marginal zone B-cell lymphomas include a number of extranodal B-cell lymphomas defined as extranodal marginal zone B-cell lymphomas of MALT type in the Revised European-American Classification of Lymphoid neoplasms (REAL classification) (3) and in the new WHO Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissue (4).
The stomach is the most common site of extranodal marginal zone B-cell lymphomas of MALT type, however, MALT lymphomas have also been described in intestitinal tract (5, 6). The small intestine, especially ileum is the most common site of intestinal MALT lymphoma. MALT lymphomas are also found in the colon in the following order of frequency; rectum, ascending colon, transverse colon, sigmoid colon and descending colon (7). According to a recent report from Korea (8), 2.5% of all MALT lymphomas have been identified as originating from the colon. Endoscopically, there are various morphologic features in gastric MALT lymphoma, revealed as irregular geographic superficial ulcer (43.7%), diffuse mucosal nodularity (25%), erosion (18.7%), atrophy (6.3%) and mucosal elevation (6.3%) (16).
Most of the colonic lymphomas except lymphomatous polyposis present as sessile protuberant or ulcerative lesions (6, 9, 10). Colonic MALT lymphoma often appears as a single mass, the most commonly in the ileocecal valve area (10).
In the previous reports of colonic MALT lymphoma in Korea, MALT lymphomas of the colon appeared endoscopically as polyps (8, 11). We report a case of colonic MALT lymphoma not identified as a protruding mass or polyp, but as a mucosal change that was found endoscopically. It is thought that the erythematous discoloration of the appendiceal orifice and the granularity in the rectum may be the early mucosal discolorization and minimal granularity in MALT lymphoma. We think that extra caution is needed when observing for not only the mucosal morphology, but also for discolorations. We have not seen a report of colonic MALT lymphoma identified (as happened in our case) by simple mucosal discolorations.
The onset of MALT lymphoma in the stomach is preceded by the acquisition of a mucosa-associated lymphoid tissue (MALT); this is generally the result of an infection by H. pylori (12, 13). The eradication of H. pylori with antibiotics can be effectively used as the sole initial treatment of localized (i.e., confined to the gastric wall) gastric MALT lymphoma. However, antibiotic treatments seem to suppress but not eradicate the neoplastic clone, and MALT lymphoma relapses have been seen years after treatment. Molecular follow-up studies revealed the persistence of the malignant clone in more than 50% of the cases in histologic remission after the antibiotic therapy. The clinical significance of this finding is still unclear. Transient and self-limiting histologic and molecular relapses can also occur. Therefore, a careful long-term follow-up is mandatory for all of the patients who have received antibiotic treatment for H. pylori (14).
Colonic MALT lymphoma has not been well investigated as compared to stomach MALT lymphoma. The metastatic ability and sensitivity against chemotherapy of the colonic MALT lymphoma is not known. In colonic MALT lymphomas, the literature suggests that surgical resection of localized lesion may be the best choice (15). In the present case, combination of multi-agent chemotherapy and radiotherapy was effective, though a long-term follow-up is definitively needed. Because colonic MALT lymphoma presents with various features, meticulous observation is mandatory in colonoscopic evaluations. As mentioned above, we think that mucosal biopsy of the discolored but not protruding and/or ulcerative mucosa is also needed for ruling out lymphoma.
Figures and Tables
Fig. 1
Colonoscopic findings at admission. (A) Colonoscopy showing granular & reddish mucosal thickening in the rectum just above anus. (B) Colonoscopy showing reddish inflammatory mucosal swelling in the appendiceal orifice.
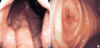
Fig. 2
Histologic findings of biopsy specimens. (A) Neoplastic lymphoid cells infiltrated colonic gland resembling a lymphoepithelial lesion (H&E, ×200). (B) Immunohistochemical stain for CD 20 showing diffuse reaction in the cell membrane. Lymphoepithelial lesions (arrow heads) are also positive for CD20 (×200).

References
1. Isaacson PG, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue: A distinctive type of B-cell lymphoma. Cancer. 1983. 52:1410–1416.

2. The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood. 1997. 89:3909–3918.
3. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink HK, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994. 84:1361–1392.
4. Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the clinical advisory committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 1999. 10:1419–1432.
5. Thieblemont C, Berger F, Dumontet C, Moullet I, Bouafia F, Felman P, Salles G, Coiffier B. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000. 95:802–806.

6. Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: Factors relevant to prognosis. Gastroenterology. 1992. 102:1628–1638.

7. d'Amore F, Brincker H, Gronbaek K, Thorling K, Pedersen M, Jensen MK, Andersen E, Pedersen NT, Mortensen LS. Non-Hodgkin's lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features and prognosis. Danish Lymphoma study group. J Clin Oncol. 1994. 12:1673–1684.
8. Hahn JS, Kim YS, Lee YC, Yang WI, Lee SY, Suh CO. Eleven-year experience of low grade lymphoma in Korea (based on REAL classification). Yonsei Med J. 2003. 44:757–770.

9. Shepherd NA, Hall PA, Coates PJ, Levinson DA. Primary malignant lymphoma of the colon and rectum. A histopathological and immunohistochemical analysis of 45 cases with clinicopathological correlations. Histopathology. 1988. 12:235–252.

10. Schmid C, Vazquez JJ, Diss TC, Isaacson PG. Primary B-cell mucosa associated lymphoid tissue lymphoma presenting as a solitary colorectal polyp. Histopathology. 1994. 24:357–362.
11. Park JS, Kim WH, Baek HJ, Kim SH, Kim BS, Woo SG, Park JH, Kim IS. A case of mucosa-associated lymphoid tissue (MALT) lymphoma of the large intestine diagnosed by sigmoidoscopy. Korean J Gastrointest Endosc. 2001. 233:122–126.
12. Zucca E, Bertoni F, Roggero E, Cavalli F. The gastric marginal zone B-cell lymphoma of MALT type. Blood. 2000. 96:410–419.

13. Zucca E, Roggero E, Pileri S. B-cell lymphoma of MALT type: a review with special emphasis on diagnostic and management problems of low-grade gastric tumours. Br J Haematol. 1998. 100:3–14.

14. Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep. 2004. 3:11–16.
15. Takada M, Ichihara T, Fukumoto S, Nomura H, Kuroda Y. Laparoscopy-assisted colon resection for mucosa-associated lymphoid tissue (MALT) lymphoma in the cecum. Hepatogastroenterology. 2003. 50:1003–1005.
16. Rhee JC, Lee HY, Rhee PL, Kim JJ, Paik SW, Koh KC, Go JH. Endoscopic findings of gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Korean J Gastrointest Endosc. 1997. 17:125–131.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download