Abstract
Emery-Dreifuss muscular dystrophy (EDMD) and limb-girdle muscular dystrophy type 1B (LGMD1B) are characterized by cardiac dysrhythmias, late-onset cardiomyopathy, slowly progressive skeletal myopathy and contractures of the neck, elbows and ankles. The causative mutation is either in the emerin gene (X-linked recessive EDMD) or lamin A/C gene (autosomal dominant EDMD2 or LGMD1B). We report three cases of EDMD, EDMD2 and LGMD1B. A 14-yr-old boy showed limitation of cervical flexion and contractures of both elbows and ankles. Sinus arrest with junctional escape beats was noted. He was diagnosed as X-linked recessive EDMD (MIM 310300). A 28-yr-old female showed severe wasting and weakness of humeroperoneal muscles. Marked limitation of cervical flexion and contractures of both elbows and ankles were noted. Varying degrees of AV block were noted. She was diagnosed as autosomal dominant EDMD2 (MIM 181350). A 41-yr-old female had contractures of both ankles and limb-girdle type muscular dystrophy. ECG revealed atrial tachycardia with high grade AV block. She was diagnosed as autosomal dominant LGMD1B (MIM 159001). Cardiac dysrhythmias in EDMD and LGMD1B include AV block, bradycardia, atrial tachycardia, atrial fibrillation, and atrial standstill, causing sudden death necessitating pacemaker implantation. Cardiologists should know about these unusual genetic diseases with conduction defects, especially in young adults.
Emerin and lamins are members of the intermediate filament protein family, and they are major components of the nuclear envelope. Mutations in the emerin gene cause X-linked recessive (XR) Emery-Dreifuss muscular dystrophy (1, 2) (EDMD: MIM 310300).
Mutations in the lamin A/C (LMNA) gene, encoding lamins A and C by alternative splicing, have been found to cause at least four different kinds of genetic disorders; autosomal dominant (AD) Emery-Dreifuss muscular dystrophy (3) (EDMD2: MIM 181350); limb-girdle muscular dystrophy type 1B (4) (LGMD1B; MIM 159001); dilated cardiomyopathy type 1A (5) (CMD1A; MIM 115200); and familial partial lipodystrophy (6) (FPLD; MIM 151660).
Among these genetic disorders, three disorders, XR EDMD, AD EDMD2 and LGMD1B, present with both cardiac and skeletal manifestations, which include cardiac dysrhythmias, late-onset cardiomyopathy, slowly progressive skeletal myopathy and contractures of the neck, elbows, and ankles. In particular, XR EDMD and AD EDMD2 share clinically undistinguishable features (7).
In these three disorders, cardiac dysrhythmias including AV block, bradycardia, atrial fibrillation, and characteristic atrial standstill often lead to syncope and sudden cardiac death. Therefore, early diagnosis and implantation of a pacemaker are life saving. Here, we report three cases of EDMD, EDMD2, and LGMD1B diagnosed by clinical findings and genetic analyses. These three patients are the first cases of EDMD, EDMD2 and LGMD1B, respectively, reported in Korea.
Three patients were examined at regular intervals over a period of three to five years by at least one of the authors. The diagnosis of these patients was made on the basis of clinical symptoms, family histories, physical as well as neurological examinations, cardiologic investigations including 12-lead electrocardiography (ECG), ambulatory ECG monitoring, electrophysiologic study and echocardiography, electromyography (EMG), laboratory studies of muscle enzymes, and muscle biopsy. Information on deceased family members as well as on other family members who were suspected to be affected was obtained from probands. Genetic analyses were performed to identify mutations in the LMNA and emerin genes. The genetic analysis of mutations in the lamin gene (EDMD2, LGMD1B) has been reported previously (8), and parts of clinical findings of case 1 and 2 were described elsewhere (9, 10).
Clinical and pathologic findings of three patients are shown in Table 1. Cardiac findings are summarized in Table 2.
A 14-yr-old boy presented with a gait disturbance. At age 1, contractures of the Achilles tendon were noted and contractures of the elbows were observed at age 9. On physical examination, the patient showed contractures of both ankles, elbow joints and limitation of neck flexion (Fig. 1A). He also showed a mild waddling and toe gait. He had weakness of the proximal upper limbs. Both proximal and distal weakness was present in the lower limbs. No deep tendon reflexes could be elicited at both ankles and elbow joints. A family history revealed that his maternal uncle had abnormal clinical features similar to those of the patient (Fig. 2A).
Serum creatine kinase (CK) activity was 481 IU/L (reference interval; 24-204 IU/L). Serum lactate dehydrogenase (LDH) level was 659 IU/L (reference interval; 240-480 IU/L). EMG revealed abnormal spontaneous activities, short duration low amplitude motor units and short duration polyphasic motor unit potentials (MUP) and early recruitment patterns on volition, suggesting myogenic disorder.
Echocardiographic findings showed normal LV systolic function and dilated right atrium and right ventricle. ECG showed sinus arrest with junctional escape rhythm (Fig. 3A). Ambulatory ECG monitoring revealed episodes of atrial fibrillation with complete AV block and junctional escape rhythm (Fig. 4A). Asystole up to 4 sec was observed. In an electrophysiologic study, no electrical signal was noted in the upper right atrium (Fig. 5). Junctional escape beats were noted. A VVI type permanent pacemaker was implanted.
Genetic analysis revealed a mutation in exon 2 of the emerin gene by deletion of the C nucleotide at position 397 of the genomic DNA sequence.
A 28-yr-old female presented to the cardiology department complaining of intermittent dizziness and palpitation. Examination showed marked wasting and weakness of the shoulder girdle, proximal arm, and the proximal and distal leg muscles. She developed progressive muscle wasting of the lower extremities and difficulty in walking during her teens. She also presented with marked limitation of cervical flexion, contracture of the both elbows limiting arm extension, and contracture of the Achilles tendon causing equinus deformities of the feet (Fig. 1B). The deep tendon reflexes of the lower legs were absent. Her elder sister died of unknown causes at 28 yr of age with similar clinical features (Fig. 2B).
Serum CK level was 195 IU/L and serum LDH level was 664 IU/L. EMG revealed increased insertional activities, short duration polyphasic MUP, and an early recruitment or decreased interference pattern, suggesting a chronic myogenic disorder. Biopsy of the left gastrocnemius muscle revealed findings compatible with muscular dystrophy (Fig. 6A). Myofibers are reduced and replaced by fibroadipose tissues. There are marked variations in fiber size associated with atrophic or hypertrophic myofibers, fiber splitting and an increase of internal nuclei. Fiber necrosis and infiltration of inflammatory cells were not present.
Echocardiography showed normal LV cavity size and normal LV systolic function, but restrictive physiology was noted. ECG showed sinus pause and first degree AV block (Fig. 3B). Slow non-sustained atrial tachycardia at a rate of 120 beats/min with high grade AV block was noted by ambulatory ECG monitoring (Fig. 4B). A VVI type permanent pacemaker was implanted due to risk of sudden death associated with high degree AV block.
Genetic analysis showed a G to A transition in exon 4 region of the lamin A/C (LMNA) gene (c.746G>A: R249Q).
A 41-yr-old female presented with ankle contractures starting in her early teens. The contractures were more severe on the right side (Fig. 1C). She had gait disturbance and difficulty climbing stairs due to ankle contractures as well as proximal leg weakness. A neurological evaluation revealed limbgirdle muscle weakness, particularly in the proximal arms and thighs. The deep tendon reflexes of both ankle joints were absent. At age 39, she experienced loss of consciousness for 30 min. One year later, transient right hemiparesis developed. Two elder sisters died at 20 and 38 yr of age, due to heart problems. Another sister had a clinical picture similar to that of the patient (Fig. 2C).
Serum CK level was 55 IU/L and serum LDH level was 618 IU/L. EMG revealed low amplitude short duration polyphasic MUP on volition and decreased interference patterns, compatible with chronic myogenic disorder. A muscle biopsy from the quadriceps muscle showed an increase in the number of internal nuclei, mild variation in the muscle fiber diameter, scattered small-angulated myofibers and fatty ingrowth (Fig. 6B). There was marked atrophy of type 1 fibers with a predominance of type 2 fibers (Fig. 6C, D).
Echocardiographic findings showed dilated cardiomyopathy. The ejection fraction of the left ventricle was 40% and end diastolic diameter of the left ventricle was 58 mm. Serial checks of ECG revealed progression of abnormalities from sinus bradycardia, first-degree AV block, to episodes of paroxysmal atrial tachycardia. Her final ECG (Fig. 3C) and ambulatory ECG monitoring showed atrial tachycardia and high grade (3:1, 4:1) AV block (Fig. 4C). A VVI type permanent pacemaker was implanted.
This patient has a G to T transversion in exon 6 region of LMNA gene (c.1130G>T: R377L).
The disorders manifested with both cardiac involvement and skeletal muscular dystrophy can be categorized into two groups. The first group is characterized by muscular dystrophy with predominant cardiomyopathy, which includes Duchenne or Becker's muscular dystrophy. The second group is characterized by muscular dystrophy with predominant cardiac conduction disturbances, which includes EDMD and LGMD1B. Both EDMD and LGMD1B are caused by the mutations of nuclear envelope proteins, i.e. emerin and lamin, respectively. EDMD consists of two diseases, XR emerin gene mutation (EDMD) and AD lamin gene mutation (EDMD2). Even though they are caused by mutations of different genes, they show very similar clinical features. LGMD1B is an allelic variant of the lamin gene mutation, which has an overlapping phenotype with EDMD (4).
It is not well-known why defects of nuclear envelope proteins show cardiac and skeletal myopathy and involvement of conduction systems in the heart. Emerin is localized at the inner nuclear membrane in various tissues by its transmembrane domain at the C-terminus (1). Amino acid sequence similarities and cellular location suggested that emerin is a member of nuclear lamina-associated protein (LAP) family. Cartegni et al. (11) proposed a general role for emerin in membrane anchorage to the cytoskeleton. In heart, its specific localization to desmosomes and fasciae adherentes of the intercalated discs could account for the characteristic conduction defects described in patients with EDMD. A deficiency of emerin in immunofluorescent staining of skeletal and cardiac muscles from EDMD patients was observed (2). The lamin A/C (LMNA) gene encodes two proteins of the nuclear lamina, lamins A (664 amino acids) and C (572 amino acids) produced by alternative splicing (5). Like emerin, lamins A and C are components of the nuclear envelope but are located in the lamina, a multicentric structure associated with the nucleoplasmic surface of the inner nuclear membrane. According to Hutchinson et al. (12), the nuclear membrane damage and lamina fragility could develop into physical cell disruption leading to myocyte death and tissue damage. In cardiac muscle, the loss of individual mononucleated myocytes is cumulative and eventually leads to atrioventricular block and heart failure when the number of affected myocytes is sufficient to cause the phenotype. Therefore, the extension of myocyte damage in nodal versus left ventricular myocardium could partly explain the earlier occurrence of atrioventricular block than left ventricular dysfunction (13).
The classical phenotype of EDMD is characterized by the clinical triad of early contractures of the Achilles tendons, elbows and postcervical muscles and slowly progressive muscle wasting and weakness with a distinctive humeroperoneal distribution in the early stages of the disease, cardiomyopathy with life threatening conduction defects (14). LGMD1B is characterized by a slowly progressive weakness of the proximal muscles, mild joint contractures, age-related atrioventricular cardiac conduction disturbances and dilated cardiomyopathy (15). Phenotypes of LGMD1B overlap with EDMD.
Although the clinical feature of muscle involvement in both XR EDMD and AD EDMD2 are quite similar, the pattern of muscular involvement in AD EDMD2 is extremely variable. In XR EDMD, the first symptoms are generally contractures, weakness and difficulty in running in patients with AD EDMD2 and are usually presented between 3 and 6 yr of age, and the contractures generally appeared afterwards. Some AD EDMD2 patients lose ambulation, unlike patients with XR EDMD in whom loss of ambulation is very rare. Therefore, muscle weakness and disease course tend to be more severe in AD EDMD2 than XR EDMD (7). In LGMD1B, symmetrical weakness starts in the proximal lower limb muscles, and gradually the upper limb muscles also become affected. Early contractures of the spine were absent, and contractures of the elbows and the Achilles tendons were either minimal or late, distinguishing this disorder from EDMD (15).
The findings of muscular involvement in our cases are compatible with the above description. In the XR EDMD patient (case 1), contractures of the Achilles tendon and the elbows were prominent without evident muscle wasting. However, AD EDMD2 (case 2) showed severe muscle wasting, difficulty in walking and joint contractures. In LGMD1B (case 3), the predominant feature is a weakness of hip girdle muscle and shoulder girdle muscles. Ankle contractures started later and milder compared with cases of EDMD.
Cardiac involvement in EDMD may occur at any age or may even be present at every onset, while on the other hand, neuromuscular symptoms precede cardiological symptoms in nearly all patients with LGMD1B (15).
The cardiac conduction defect is the most serious and life threatening clinical manifestation of the disease and is considered to be a hallmark of cardiomyopathy of EDMD. ECG in the early findings shows sinus bradycardia, small P-wave and prolonged P-R interval. According to the progress of disease, ECG shows atrial fibrillation or flutter, complete AV block with idioventricular rhythm and atrial paralysis (atrial standstill) (14). The documentation of the absence of electrical atrial activity and inability to pace the atria by electrocardiogram and electrophysiological study in a patient with atrial paralysis, is recognized as a pathognomonic finding of EDMD (16). The normal myocardium is progressively replaced by fibroadipose tissue (17), which results in atrial paralysis by loss of atrial contractility and heart failure by atrial and ventricular dilatation. Carriers are also at risk of cardiac arrhythmias and sudden death (17). Patients with LGMD1B show similar cardiological abnormalities to those of EDMD. They include conduction disturbances and dysrythmias, presenting as an AV block, atrial fibrillation with high degree AV block and junctional escape rhythm, bradycardia, atrial standstill and syncopal attacks. AV conduction disturbances increased with age in LGMD1B patients and became more severe (15). The potential complications of the cardiac pathology in EDMD and LGMD1B include syncope, cerebral and pulmonary emboli, congestive heart failure, cor pulmonale, and sudden cardiac death, which may occur in the early twenties (18).
In our study, the manifestations of cardiac involvement were similar to those of reported cases. Atrial standstill was noted in patients with EDMD and LGMD1B. The patient with LGMD1B had a history of syncope and cerebral thromboembolism. The patient with LGMD1B showed findings of dilated cardiomyopathy. However, in patients with EDMD, the left ventricular function was preserved. All three patients had severe bradycardia due to either conduction defects, atrial tachycardia with high grade AV block, atrial fibrillation, and sinus arrest with junctional escape rhythm.
Merlini et al. (19) reviewed 73 cases of XR EDMD from six families, and showed that 30 patients died suddenly between the age of 25 and 59 yr. Among them, only four patients had minor symptoms with dizziness or syncopal episodes, therefore, they ascertained that sudden death due to EDMD was unpredictable in most cases. Consequently, a very important aspect of management of EDMD and LGMD1B was determined to be the early detection of cardiac conduction defects. The insertion of a pacemaker may then be life saving. In our cases, all patients were implanted with pacemakers. However, these patients had not been diagnosed before, even though they were consulted to cardiologists for rhythm disturbances in other hospitals.
Based on the experience of our cases, we propose that screening for mutations in the emerin or lamin gene should be considered in patients with severe conduction defects and/or dilated cardiomyopathy. Vohanka et al. (20) reported that screening for mutations in the XR EDMD gene was performed among 450 patients affected with severe heart rhythm defects and/or dilated cardiomyopathy, and XR EDMD was found among ten patients. Therefore, investigators should be concerned about the genetic association of severe cardiac involvement even in patients without muscular symptoms.
In conclusion, EDMD and LGMD1B are characterized by slowly progressive skeletal myopathy and contractures of neck, elbows and ankles, cardiac dysrhythmia, and late-onset cardiomyopathy. Cardiac dysrhythmias in EDMD and LGMD1B include AV block, bradycardia, atrial fibrillation, sinus arrest and characteristic atrial standstill, causing syncope and sudden cardiac death necessitating pacemaker implantation. Because these patients are commonly undiagnosed, cardiologists should be aware of these unusual genetic diseases as causes of conduction defects, especially in young adults.
Figures and Tables
 | Fig. 1Photographs of each patient. (A) XR EDMD patient (case 1) showing flexion contractures of both elbows and Achilles tendons. (B) AD EDMD2 patient (case 2) showing marked muscular atrophy of upper and lower extremities with flexion contractures of both elbows and Achilles tendons. The patient keeps her heel off the floor with equinus deformities of the feet. (C) LGMD1B patient (case 3) shows peroneal dystrophy with Achilles tendon contractures. Equinus deformity of the right foot is more severe than the left one. |
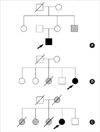 | Fig. 2Pedigrees of the families with XR EDMD (A), AD EDMD2 (B) and LGMD1B (C). Squares indicate male family members, circles female family members and symbols with a slash mark deceased family members. Open symbols indicate unaffected family members, filled symbols indicate the presence of neuromuscular and cardiac symptoms, and shaded symbols indicate family members who are or were probably affected. Arrows represent proband. B and C from reference 8. |
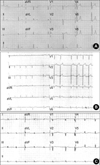 | Fig. 312-lead ECG of EDMD (A), AD EDMD2 (B) and LGMD1B (C). (A) The ECG shows sinus arrest with junctional escape rhythm at a heart rate of 40 beats per minute. (B) The ECG shows left axis deviation, left anterior hemiblock, first-degree atrioventricular block, and sinus pause. (C) The ECG shows atrial fibrillation with ST-T changes. |
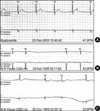 | Fig. 4Findings of ambulatory ECG monitorings of EDMD (A), AD EDMD2 (B) and LGMD1B (C). (A) Atrial fibrillation with complete AV block and junctional escape rhythm. (B) Slow nonsustained atrial tachycardia at a rate of 120 beats per minute with high grade AV block. (C) Atrial tachycardia and high grade (3:1, 4:1) AV block with the longest R-R interval of 2.06 sec. |
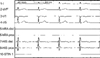 | Fig. 5Findings of electrophysiologic study in a patient with XR EDMD (case 1). Electrophysiologic study shows no electrical signal in the right upper atrium. Junctional escape beats are noted. |
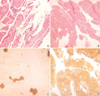 | Fig. 6The histopathologic findings of skeletal muscle biopsy specimen in AD EDMD2 (A) and LGMD1B (B-D) patient. (A) Myofibers are reduced in number and replaced by fibroadipose tissues. There are atrophic or hypertrophic myofibers, internalization of sarcolemmal nuclei and fiber splitting (hematoxylin and eosin stain, ×200). (B) Variation in myofiber size, frequent internal nuclei and fatty ingrowth are noted in hematoxylin and eosin stain (×200). (C) ATPase pH 4.6 stain reveals atrophy of type 1 fibers (dark brown stain, ×200). (D) ATPase pH 9.4 stain reveals a predominance of type 2 fibers (brown stain, ×100). |
ACKNOWLEDGMENTS
We are deeply indebted to Dr. Manfred Wehnert (Institute of Human Genetics, Ernst-Moritz-Arndt University Greifswald, Germany) for genetic analysis of mutation in the emerin gene.
References
1. Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994. 8:323–327.

2. Nagano A, Koga R, Ogawa M, Kurano Y, Kawada J, Okada R, Hayashi YK, Tsukahara T, Arahata K. Emerin deficiency at the nuclear membrane in patients with Emery-Dreifuss muscular dystrophy. Nat Genet. 1996. 12:254–259.
3. Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K. Mutation in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999. 21:285–288.
4. Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet. 2000. 9:1453–1459.

5. Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ Jr, Spudich S, de Girolami U, Seidman JG, Seidman C, Muntoni F, Muehle G, Johnson W, McDonough B. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999. 341:1715–1724.

6. Shackleton S, Lloyd D, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O'Rahilly S, Trembath RC. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000. 24:153–156.

7. Bonne G, Mercuri E, Muchir A, Urtizberea A, Becane HM, Recan D, Merlini L, Wehnert M, Boor R, Reuner U, Vorgerd M, Wicklein EM, Eymard B, Duboc D, Penisson-Besnier I, Cuisset JM, Ferrer X, Desguerre I, Lacombe D, Bushby K, Pollitt C, Toniolo D, Fardeau M, Schwartz K, Muntoni F. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann Neurol. 2000. 48:170–180.

8. Ki CS, Hong JS, Jeong GY, Ahn KJ, Choi KM, Kim DK, Kim JW. Identification of lamin A/C (LMNA) gene mutations in Korean patients with autosomal dominant Emery-Dreifuss muscular dystrophy and limb-girdle muscular dystrophy 1B. J Hum Genet. 2002. 47:225–228.

9. Hong JS, Kang JH, Lee GS, Lee CS, Choi HJ, Lee BD, Kim JS, Suh YL, Kim DK, Chi JG, Ahn KJ. A case of high degree AV block treated by implantation of permanent pacemaker in Emery-Dreifuss muscular dystrophy. Korean Circ J. 2000. 30:1316–1322.

10. Kim SE, Hong JS, Ahn KJ, Kim JS, Kim DK. A case of Emery-Dreifuss muscular dystrophy by emerin gene mutation. Korean Circ J. 2003. 33:143–149.

11. Cartegni L, di Barletta MR, Barresi R, Squarzoni S, Sabatelli P, Maraldi N, Mora M, Di Blasi C, Cornelio F, Merlini L, Villa A, Cobianchi F, Toniolo D. Heart-specific localization of emerin: new insights into Emery-Dreifuss muscular dystrophy. Hum Mol Genet. 1997. 6:2257–2264.

12. Hutchison CJ, Alvarez-Reyes M, Vaughan OA. Lamins in disease: why do ubiquitously expressed nuclear envelope proteins give rise to tissue-specific disease phenotypes? J Cell Sci. 2001. 114:9–19.

13. Arbustini E, Pilotto A, Repetto A, Grasso M, Negri A, Diegoli M, Campana C, Scelsi L, Baldini E, Gavazzi A, Tavazzi L. Autosomal dominant dilated cardiomyopathy with atrioventricular block: A lamin A/C defect-related disease. J Am Coll Cardiol. 2002. 39:981–990.

14. Emery AE. Emery-Dreifuss muscular dystrophy - a 40 year retrospective. Neuromuscul Disord. 2000. 10:228–232.

15. van der Kooi AJ, Ledderhof TM, de Voogt WG, Res CJ, Bouwsma G, Troost D, Busch HF, Becker AE, de Visser M. A newly recognized autosomal dominant limb girdle muscular dystrophy with cardiac involvement. Ann Neurol. 1996. 39:636–642.

16. Marshall TM, Huckell VF. Atrial paralysis in a patient with Emery-Dreifuss muscular dystrophy. Pacing Clin Electrophysiol. 1992. 15:135–140.

17. Fishbein MC, Siegel RJ, Thompson CE, Hopkins LC. Sudden death of a carrier of X-linked Emery-Dreifuss muscular dystrophy. Ann Intern Med. 1993. 119:900–905.

18. Zacharias AS, Wagener ME, Warren ST, Hopkins LC. Emery-Dreifuss muscular dystrophy. Semin Neurol. 1999. 19:67–79.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download