Abstract
We aimed to examine the effects of angiotensin II AT1 receptor blocker on the expression of major renal sodium transporters and aquaporin-2 (AQP2) in rats with chronic renal failure (CRF). During 2 wks after 5/6 nephrectomy or sham operation, both CRF rats (n=10) and sham-operated control rats (n=7) received a fixed amount of low sodium diet and had free access to water. CRF rats (n=10) were divided into two groups which were either candesartan-treated (CRF-C, n=4) or vehicletreated (CRF-V, n=6). Both CRF-C and CRF-V demonstrated azotemia, decreased GFR, polyuria, and decreased urine osmolality compared with sham-operated rats. When compared with CRF-V, CRF-C was associated with significantly higher BUN levels and lower remnant kidney weight. Semiquantitative immunoblotting demonstrated decreased AQP2 expression in both CRF-C (54% of control levels) and CRF-V (57%), whereas BSC-1 expression was increased in both CRF groups. Particularly, CRF-C was associated with higher BSC-1 expression (611%) compared with CRF-V (289%). In contrast, the expression of NHE3 (25%) and TSC (27%) was decreased in CRF-C, whereas no changes were observed in CRF-V. In conclusion, 1) candesartan treatment in an early phase of CRF is associated with decreased renal hypertrophy and increased BUN level; 2) decreased AQP2 level in CRF is likely to play a role in the decreased urine concentration, and the downregulation is not altered in response to candesartan treatment; 3) candesartan treatment decreases NHE3 and TSC expression; and 4) an increase of BSC-1 is prominent in candesartan-treated CRF rats, which could be associated with the increased delivery of sodium and water to the thick ascending limb.
It has been demonstrated that when total renal mass is reduced in chronic renal failure (CRF), single nephron glomerular filtration rate (SNGFR) in the remaining nephrons markedly increases, thereby leading to an increased reabsorptive burden on the residual nephrons (1). This together with the compensatory renal hypertrophy is associated with significant alterations in tubular reabsorptive capacities of sodium and water. In CRF, there is an adaptive increase in sodium excretion by each residually functioning nephron (2, 3). In this way, sodium balance can be maintained despite a diminishing GFR when intake of salt and water is unaltered in CRF. The mechanisms responsible for the increased fractional sodium excretion and marked natriuresis per nephron in remnant kidney have previously been investigated (3-5). In particular, we have previously performed a profiling study in rats with CRF (6, 7), demonstrating that densities per nephron of type 3 Na/H exchanger (NHE3), type-2 Na-Pi cotransporter (NaPi-2), and Na-K-ATPase (alpha1-subunit) do not increase proportionately to the extensive nephron hypertrophy, associated with the hyperfiltration in remnant nephrons. In contrast, the densities per nephron of both rat type 1 bumetanide-sensitive Na-K-2Cl cotransporter (BSC-1) in the thick ascending limb (TAL) and thiazide-sensitive Na-Cl cotransporter (TSC) in the distal convoluted tubule (DCT) were significantly increased. Also, there was no decrease in Na-K-ATPase immunolabeling in TAL and DCT. Thus, the expression of sodium transporters in the TAL and DCT was not decreased in remnant kidneys, indicating that the altered tubular handling of sodium in CRF may be caused primarily by the changes in expression levels of proximal tubule sodium transporters and that there appears to be a compensatory increase in sodium transporter expression in the distal nephron.
It has been demonstrated that renin-angiotensin-aldosterone (RAA) system is activated in CRF and the key role of AngII in the progression of CRF has been revealed in several studies (8, 9). We have previously demonstrated that chronic infusion of supraphysiological doses of AngII in normal rats was associated with significantly increased abundance and apical expression level of the Na-H exchanger NHE3 and Na-K-2Cl cotransporter BSC-1 in medullary thick ascending limbs (mTAL), whereas Na,K-ATPase and electroneutral Na-HCO3 cotransporter (NBCn1) levels remained unchanged (10). In the proximal tubule of AngII-treated rats, the overall abundance of NHE3 and Na,K-ATPase remained unchanged. In contrast immunoperoxidase microscopy and confocal laser scanning microscopy demonstrated that NHE3 immunolabeling in the brush border of the proximal tubules was markedly enhanced in AngII-treated rats, suggesting that high plasma AngII levels may contribute to an enhanced renal Na+ and HCO3- reabsorption in kidney tubules by increasing the abundance of NHE3 and BSC-1 in mTAL cells as well as increasing NHE3 expression in the proximal tubule brush border. Therefore, the activated RAA system in parallel to the changes of SNGFR and renal hypertrophy in CRF could be involved in the changes of renal sodium handling and of the expression of renal sodium transporters.
Two main subtypes of the Ang II receptor, AT1 and AT2, have been characterized. Most of the physiological and pathophysiological actions of AngII are mediated by the AT1 receptor, and AT1 receptors can be blocked by specific AT1 receptor antagonists. Compared to ACE inhibitors, AT1 receptor antagonists inhibit the RAA system more specifically blocking the effects of AngII at the receptor level without interfering with the bradykinin metabolism. In the present study, we aimed to examine the effects of AngII AT1 receptor blockade on the expression of major renal sodium transporters (NHE3, BSC-1, and TSC) and vasopressin-regulated aquaporin-2 (AQP2) in rats with an early stage of CRF induced by 5/6 nephrectomy. This would be an extension of our previous studies (6, 7, 10, 11) and could further elucidate the role of AngII on the expression of renal sodium transporters in the kidney tubule segments.
The studies were performed on adult male Sprague-Dawley rats initially weighing 200-210 g (Hyochang Science, Daegu, Korea). The rats were maintained on a standard rodent diet with free access to water before the experiments.
Experimental CRF was induced by excision of about 2/3 of the right kidney and left total nephrectomy, using the so-called excision remnant kidney model (6, 7). The protocols used in this study are depicted in Fig. 1. The rats were anesthetized with isoflurane (Choongwae Pharma Corporation, Seoul, Korea) and during the surgery the rats were placed on a heated table to maintain rectal temperature at 37-38℃. The right kidney was exposed through right flank incision, gently dissected free from the adrenal gland, and approximately two-thirds of the right kidney including the upper and lower pole was excised. One week later, these rats were again anesthetized with isoflurane, the left kidney was removed through left flank incision after dissecting it free from the adrenal gland. The wound was closed with 4/0 silk and metal clamps. Immediately after each surgical procedure, rats were allowed to recover from anesthesia and surgery in individual cages. As a control group, rats were subjected to sham operation identical to the ones used for CRF rats except that kidneys or poles of kidney were not removed. Sham-operated rats were monitored for two weeks in parallel with 5/6 nephrectomized rats (Fig. 1). All rats were killed under isoflurane anesthesia and kidneys were rapidly removed and processed for protein preparation at the same day. We have chosen 2 week-follow-up after induction of 5/6 nephrectomy to 1) produce chronic renal insufficiency, 2) to allow significant hypertrophy of the remnant kidney, and 3) to minimize interstitial fibrosis, as previously demonstrated (6, 7).
During the 2 weeks after 5/6 nephrectomy and sham operation, all CRF rats and sham-operated rats received a fixed amount (15 g/220 g bw/day) of low sodium diet (TD 90228, Harlan Teklad, Madison, WI, which the supplier indicated 0.02% Na+) to supply same amount of sodium intake and to increase endogenous angiotensin II and aldosterone levels in both CRF rats and sham-operated control rats, as previously demonstrated (12, 13). CRF rats (n=10) were divided into two groups which were either vehicle-treated (CRF-V, n=6) or candesartan-treated (CRF-C, 1 mg/kg/day for 2 wks, s.c., n=4). The estimated daily sodium intake in food per rat was 0.13 mEq Na+/220 g bw/day*. They had free access to water intake. For candesartan infusion to CRF rats, osmotic minipumps (Model 2002, Alzet Co, Palo Alto, CA, U.S.A.) were implanted subcutaneously in the neck of each rat (6). For implantation, osmotic minipumps were filled with candesartan (AT1 receptor blocker, a gift from the AstraZeneca Pharmaceutical, Sweden) dissolved in 0.02M Na2CO3 in physiologic saline (14). The pumps were equilibrated with physiologic saline at 37℃ for 4 hr before insertion. The dose of candesartan (when given in drinking water) has been shown to be sufficient to block the rise in blood pressure resulting from long-term infusion of angiotensin II (15).
The rats were maintained in the metabolic cages during the specific days indicated as asterisks in Fig. 1, allowing quantitative urine collections and measurements of water intake. Urine volume, osmolality, and urine creatinine concentration were measured. Blood was collected from the abdominal aorta at the time of sacrifice for measurement of serum sodium and potassium concentration, creatinine, and osmolality. Urine sodium measurement could not be obtained since the concentration was at a very low level to detect in an automatic analyzer.
All rats were killed under light isoflurane anesthesia and right kidneys were rapidly removed and were homogenized (Ultra-Turrax T8 homogenizer, IKA Labortechnik, Staufen, Germany) in ice-cold isolation solution containing 0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, 8.5 µM leupeptin, 1 mM phenylmethylsulfonyl fluoride, pH 7.2. To remove whole cells, nuclei and mitochondria, the homogenates were centrifuged at 4,000 g for 15 min at 4℃ and the supernatant was pipetted off and kept on ice for further processing. The total protein concentration was measured (Pierce BCA protein assay reagent kit, Pierce, Rockford, IL, U.S.A.) and all samples were adjusted with isolation solution to reach the identical final protein concentrations (for this study, we adjusted the final protein concentration of each kidney from CRF rats and sham-operated control rats to 10 µg/µL) and solubilized at 65℃ for 15 min in Laemmli sample buffer, and then stored at -20℃. To confirm equal loading of protein, an initial gel was stained with Coomassie blue dye, as described previously (10). SDS-PAGE was performed on 9% or 12% polyacrylamide gels. The proteins were transferred from the gel electrophoretically (BioRad Mini Protean II) to nitrocellulose membranes (Hybond ECL RPN3032D, Amersham Pharmacia Biotech, Little Chalfont, U.K.). After transfer the blots were blocked with 5% milk in PBS-T (80 mM Na2HPO4, 20 mM Na2HPO4, 100 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 hr and incubated overnight at 4℃ with primary antibodies. Immunoblotting was performed using anti-rat AQP2 (7), anti-rat NHE3 (6, 10), anti-rat BSC-1 (6, 10), and anti-rat TSC antibodies (6, 10). The sites of antibody-antigen reaction were visualized with horseradish peroxidase (HRP)-conjugated secondary antibodies (P448, diluted 1: 3,000; DAKO, Glostrup, Denmark) with an enhanced chemiluminescence (ECL) system and exposure to photographic film (Hyperfilm ECL, RPN3103K, Amersham Pharmacia Biotech, Little Chalfont, U.K.). The band densities were quantitated by scanning the films and the density was calculated as a fraction of the mean control value for that gel.
Urine output was significantly increased after induction of 5/6 nephrectomy in CRF rats (both CRF-V and CRF-C), whereas there was no change in urine output after sham operation in control rats (Fig. 2, Table 1). The urine output was similar between CRF-V and CRF-C (Fig. 2, Table 1), indicating that the high urine output seen in rats with CRF was not affected by the candesartan treatment. Consistent with the increased urine output in CRF, the urine osmolality was significantly decreased in both CRF-V and CRF-C, compared with sham-operated rats (Table 1).
BUN levels were significantly increased in CRF rats (both CRF-V and CRF-C) compared with sham-operated rats, respectively (Table 1). This indicated that they had a significant azotemia (Table 1). Moreover, BUN level in CRF rats treated with candesartan was much higher than that of CRF rats treated with vehicle (153.8±9.6 in CRF-C vs. 77.2±13.9 mg/dL in CRF-V, p<0.05, Table 1). Consistent with the significant azotemia, serum creatinine level was increased and creatinine clearance was decreased in CRF rats with no difference between the two CRF groups (CRF-V vs. CRF-C, Table 1). CRF rats treated with vehicle was associated with significant hypertrophy of remnant renal mass, evidenced by the increase of kidney weight comparable to the corresponding right kidney weight of sham-operated control rats despite 2/3 nephrectomy of right kidney (0.39±0.02 in CRF-V vs. 0.36±0.004 g/100 g bw in sham-operated control rats, not significant (n.s.), Table 1). In contrast, candesartan treatment in CRF rats (CRF-C) was associated with significantly decreased kidney weight compared with vehicle-treated CRF rats (0.29±0.03 vs. 0.39±0.02 g/100 g bw, p<0.05, Table 1), suggesting that candesartan treatment in an early phase of CRF significantly reduced the renal hypertrophy and AngII could play a role in this process.
Semi-quantitative immunoblotting of whole kidney protein samples from CRF rats (remnant kidney) and sham-operated rats (corresponding right whole kidney) demonstrated that CRF rats, both vehicle-treated (57±9% of sham-operated control level, p<0.05) and candesartan-treated (54±6% of sham-operated control level, p<0.05), had significantly decreased AQP2 expression compared with sham-operated controls (Fig. 3, Table 2). In contrast, the whole kidney AQP2 expression in CRF rats was not altered in response to candesartan treatment (Fig. 3, Table 2).
Semi-quantitative immunoblotting of whole kidney protein samples from CRF rats and sham-operated rats demonstrated that CRF rats, both vehicle-treated and candesartan-treated, had significantly increased BSC-1 expression, compared with sham-operated controls (Fig. 4, Table 2). Moreover, candesartan treatment in CRF rats significantly increased whole kidney BSC-1 expression (611±126% of sham-operated control level), compared with CRF rats treated with vehicle only (289±63% of sham-operated control level, p<0.05, Fig. 4, Table 2).
Candesartan treatment significantly decreased whole kidney NHE3 expression in CRF rats (25±5% of sham-operated control level, p<0.05, Fig. 5, Table 2), whereas whole kidney NHE3 expression was unchanged in CRF rats treated with vehicle only (76±16% of sham-operated control level, n.s., Fig. 5, Table 2). Moreover, densitometric analysis revealed that candesartan treatment significantly decreased whole kidney TSC expression in CRF rats (27±13% of sham-operated control level, p<0.05, Fig. 6, Table 2), whereas whole kidney TSC expression was unchanged in CRF rats treated with vehicle only (75±16% of sham-operated control level, n.s., Fig. 6, Table 2).
In the present study, we demonstrated that 1) CRF rats treated with candesartan were associated with decreased kidney weight and increased BUN level compared with CRF rats treated with vehicle; 2) CRF rats, both candesartan-treated and vehicle-treated, demonstrated decreased AQP2 levels in remnant kidneys and this was likely to play a role in the decreased urine concentration. The decreased AQP2 expression in CRF rats was not altered in response to candesartan treatment; 3) Candesartan treatment was associated with decreased NHE3 and TSC expression in CRF, which could be due to the Ang II AT1 receptor blockade and/or decreased aldosterone levels; and 4) BSC-1 expression was increased in both CRF groups and the increased expression of BSC-1 was more prominent in candesartan-treated CRF rats compared with vehicle-treated CRF rats.
AT1 receptor blockade with candesartan treatment in CRF rats was associated with decreased kidney weight and increased BUN levels, compared with CRF rats treated with vehicle alone. This may indicate that AngII played a role in the process of renal hypertrophy as a growth factor (16) and AT1 receptor blockade prevented or reduced renal hypertrophy which is a common feature in remnant kidney. Consistent with this, recent studies have shown that AngII acts as a growth factor directly on the myocardium to cause ventricular hypertrophy, and this response was prevented or reversed by selective AT1 receptor antagonists (17). In addition, it is of an interest to examine whether the hypertrophy of renal tubular segments in remnant kidney is selectively responded in response to AT1 receptor blockade. Morphometric studies would be needed to elucidate this issue.
Urinary concentration and dilution depends on the presence of a discrete segmental distribution of transport properties along the renal tubule and urinary concentration depends on 1) a hypertonic medullary interstitium, which is generated by active NaCl reabsorption as a consequence of countercurrent multiplication in water-impermeable nephron segments; 2) a high water permeability (constitutive or vasopressin-regulated) in other renal tubular segments for osmotic equilibration, which chiefly depends on the expression of aquaporins; and 3) flow rate of tubule fluid entering the medullary collecting ducts (18, 19). Thus, defects in any of these mechanisms would be predicted to be associated with urinary concentrating defects.
The collecting duct represents the final site for the control of water excretion into the urine (18, 20). Water permeability of the collecting duct is tightly regulated, under the control of the vasopressin, which causes a dramatic increase in collecting duct water permeability, allowing reabsorption of water from the tubular fluid down an osmotic gradient. In the previous study (7), we found a marked reduction in the expression of two collecting duct water channels AQP2 and AQP3 in rats with CRF, indicating a defect in collecting duct water reabsorption. This is consistent with previous functional studies. First, in rats with CRF, micropuncture and microcatheterization studies have indicated that the impaired urinary concentrating ability may, at least partly, be caused by an impairment of vasopressin-stimulated water reabsorption in the collecting duct (21). Secondly, patients with advanced CRF have urine which remains hypotonic to plasma despite the administration of supramaximal doses of vasopressin (22). This vasopressin-resistant hyposthenuria specifically implies abnormalities in collecting duct water reabsorption in CRF patients. Thirdly, Fine et al. (23) observed that isolated and perfused cortical collecting ducts dissected from remnant kidneys of severely uremic rabbits exhibited a defect in the response to vasopressin. This was demonstrated both as a decreased water flux (Jv) and decreased adenylate cyclase activity. Importantly, they also showed that the cAMP analogue 8-bromo-cAMP failed to induce a normal hydrosmotic response in cortical collecting duct from remnant kidneys. As an extension of these observations, Teitelbaum and McGuinness (24) observed that RT-PCR of total RNA from the inner medulla of CRF rat kidneys revealed virtual absence of V2 receptor mRNA. Thus, these studies all provide firm evidence for the decreased of vasopressin-regulated water channel AQP2 expression and the significant defects in the collecting duct of the kidneys from CRF.
In the present study, we further examine whether Ang II AT1 receptor blocker candesartan treatment in rats with CRF, where plasma vasopressin and AngII levels are elevated, could alter the downregulation of renal AQP2 expression and decreased urine concentration which were already seen in CRF rats (7). In CRF, plasma vasopressin levels are already known to be elevated. Moreover, in the present study rats with CRF (both candesartan-treated and vehicle-treated) and sham-operated rats received a fixed amount of low sodium diet to increase endogenous AngII and aldosterone levels, which was previously demonstrated in several studies (12-14) and to give same amount of sodium intake. Interestingly, in a parallel study, we found that treatment with the AT1 receptor blocker candesartan in NaCl-restricted normal rats prevented the increase of the expression of inner medullary AQP2 and phosphorylated-AQP2 (AQP2 phosphorylated in the PKA-phosphorylation consensus site Ser-256) and the increase of urine concentration in response to the long-term dDAVP treatment (25). This could be possible since there is cross-talk between the signaling pathways of vasopressin and AngII (25-27). In contrast, the present study examined on CRF rats did not show any difference in total kidney AQP2 expression and urine concentration between the NaCl-restricted CRF rats treated with candesartan and NaCl-retricted CRF rats treated with vehicle alone. This is possible since CRF per se has significant defects in collecting duct function in water reabsorption and vasopressin responsiveness as discussed previously and thus this could not be like the case of normal rats.
The Na-K-2Cl cotransporter BSC-1, which is localized at the apical plasma membrane domains of medullary and cortical thick ascending limb segments, mediates the apical NaCl transport in these water-impermeable segments (6, 28). This pathway is critical for the generation of the hypertonic medullary interstitium for concentrating urine. Consistent with this, we have previously demonstrated that downregulation of BSC-1 is associated with a marked reduction of urine concentration in several well characterized animal models, e.g., PTH-induced hypercalcemia (29) and low potassium diet-induced hypokalemia (30). Moreover, it was demonstrated that the expression of Na-K-2Cl cotransporter in the thick ascending limb is increased in response to chronic oral saline loading and dDAVP treatment in rats (31).
In the present study, we demonstrated an increase of BSC-1 protein expression in CRF rats treated with vehicle compared with sham-operated rats, and this was consistent with our previous study demonstrating an increase of density per nephron of BSC-1 in CRF rats (although the previous study demonstrated unchanged protein expression of BSC-1 between CRF and sham-operated rats (6)). The delivery of sodium and water to the distal end of the proximal tubule of superficial nephrons and to the bend of Henle's loop of juxtamedullary nephrons is known to be increased in remnant kidneys due to an increase in the filtered load and a decrease in the fractional reabsorption of sodium and water in the proximal tubule (21). This condition may be similar to acute and chronic loading with isotonic saline in normal rats. A large amount of evidence indicates that isotonic saline loading is associated with an elevation of SNGFR as well as a decrease in fractional proximal NaCl reabsorption. Furthermore, Landwehr et al. (32) demonstrated that the increase in delivery to the loop of Henle is associated with a marked increase in NaCl absorption by the loop of Henle, presumably due in part to an increase in TAL NaCl absorption. Consistent with this, Ecelbarger et al. (31) demonstrated that chronic oral saline loading in rats markedly increased BSC-1 abundance in the TAL of rats. Thus, we speculate that the increase in density of BSC-1 seen following 2 weeks after induction of 5/6 nephrectomy could, at least in part, be a consequence of a prolonged increase in the NaCl load delivered to the TAL due to both hyperfiltration and altered expression in the proximal sodium transporters in remnant kidneys.
Moreover, candesartan treatment potentiated the upregulation of BSC-1 protein expression in CRF rats, and this is also compatible with the view that BSC-1 expression is regulated by the prolonged increase in the NaCl load delivered to the TAL due to both hyperfiltration and altered expression in the proximal tubule sodium transporters in remnant kidneys. Consistent with this, the protein expression of NHE3, which is mainly localized at the apical membrane domains of the proximal tubule and plays a role in the sodium reabsorption, was significantly decreased in CRF rats treated with candesartan compared with CRF rats treated with vehicle but the GFR between the two groups was not different. This could induce an increased loading of NaCl and fluid into the TAL, thereby increasing the BSC-1 expression. In contrast, mice lacking NHE3 showed decreased expression of BSC-1 and sodium wasting (33), however they also had decreased SNGFR in contrast to CRF (34). The observed decrease in BSC-1 abundance in the NHE3 knockout mice is unexplained based on current knowledge regarding the regulation of these transporters and further investigation is needed to address this.
Figures and Tables
Fig. 1
A diagram of the study design. CRF was induced in rats by right 2/3 nephrectomy followed by contralateral nephrectomy (n=10). Sham-operated control rats matching CRF rats (n=7). Both CRF rats and sham-operated control rats received a fixed amount of low sodium diet for 2 wks after induction of CRF. Two groups of CRF are made: CRF rats treated with vehicle (CRF-V, n=6) and CRF rats treated with candesartan (CRF-C, n=4). Sham-operated control rats were also treated with vehicle. The rats were maintained in metabolic cages at the days marked with asterisk (*), allowing monitoring of daily urine output and water intake. The stippled lines indicated the treatment of vehicle or candesartan in each group.

Fig. 2
Time course of the changes in urine output in CRF rats and sham-operated rats. Urine output was significantly increased after induction of 5/6 nephrectomy in CRF rats (both CRF-V (circle) and CRF-C (rectangle)), whereas there is no change in urine output after sham operation in control rats (triangle). *p<0.05 when CRF-V or CRF-C was compared with sham-operated control rats.

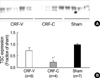
Fig. 3
Decreased expression of AQP2 in CRF-V and CRF-C. Immunoblot of whole kidney protein samples from CRF rats and sham-operated control rats. (A) The immunoblot was reacted with affinity purified anti-AQP2 and reveals 29 kDa and 35-50 kDa AQP2 bands, representing non-glycosylated and glycosylated forms of AQP2. (B) Densitometric analysis demonstrated a decrease in AQP2 expression in CRF-V and CRF-C, compared with sham-operated control rats (Sham). *p<0.05 when CRF-V or CRF-C was compared with sham-operated control rats.
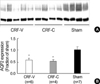
Fig. 4
Increased expression of BSC-1 in CRF-V and CRF-C. Immunoblot of whole kidney protein samples from CRF and sham-operated control rats. (A) The immunoblot was reacted with affinity purified anti-BSC-1 and recognized a broad band of molecular mass 146-176 kDa. (B) Densitometric analysis revealed a significant increase of BSC-1 expression in CRF-V and CRF-C compared with sham-operated rats (Sham). Moreover, CRF-C was associated with higher BSC-1 expression compared with CRF-V. *p<0.05 when CRF-V or CRF-C was compared with sham-operated controls, †p<0.05 when CRF-C was compared with CRF-V.
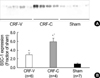
Fig. 5
Decreased expression of NHE3 in CRF-C. Immunoblot of whole kidney protein samples from CRF and sham-operated rats. (A) The immunoblot was reacted with affinity purified anti-NHE3 and revealed a single ~87 kDa band. (B) Densitometric analysis revealed a marked decrease in NHE3 expression in the CRF-C, compared with CRF-V and sham-operated control rats (Sham). *p<0.05 when CRF-V or CRF-C was compared with sham-operated controls, †p<0.05 when CRF-C was compared with CRF-V.
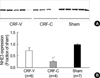
Fig. 6
Decreased expression of TSC in CRF-C. Immunoblot of whole kidney protein samples from CRF and sham-operated rats. (A) The immunoblot was reacted with affinity purified anti-TSC and revealed a broad band centered at ~164 kDa band. (B) Densitometric analysis revealed a marked decrease in TSC expression in CRF-C, compared with CRF-V and sham-operated controls. *p<0.05 when CRF-V or CRF-C was compared with sham-operated controls, †p<0.05 when CRF-C was compared with CRF-V.
Notes
*Sample calculation: 1) daily food intake in each rat: 15 g/220 g bw; 2) Na+ content in food (Harlan Teklad, TD 90228): 0.02%; 3) daily Na+ intake in rat: (15 g/220 g bw)×0.02%=0.003 g Na+/220 g bw/day; 4) 1 mmoL Na+=23 mg; 5) thus 0.003 gNa+/220 g bw/day corresponds to 0.13 mmoL Na+/220 g bw/day (0.13 mEq Na+/220 g bw/day).
References
2. Slatopolsky E, Elkan IO, Weerts C, Bricker NS. Studies on the characteristics of the control system governing sodium excretion in uremic man. J Clin Invest. 1968. 47:521–530.

3. Hayslett JP, Kashgarian M, Epstein FH. Mechanism of change in the excretion of sodium per nephron when renal mass is reduced. J Clin Invest. 1969. 48:1002–1006.

4. Trizna W, Yanagawa N, Bar Khayim Y, Houston B, Fine LG. Functional profile of the isolated uremic nephron. Evidence of proximal tubular "memory" in experimental renal disease. J Clin Invest. 1981. 68:760–767.
5. Fine LG, Trizna W, Bourgoignie JJ, Bricker NS. Functional profile of the isolated uremic nephron. Role of compensatory hypertrophy in the control of fluid reabsorption by the proximal straight tubule. J Clin Invest. 1978. 61:1508–1518.
6. Kwon TH, Frokiaer J, Fernandez-Llama P, Maunsbach AB, Knepper MA, Nielsen S. Altered expression of Na transporters NHE-3, NaPi-II, Na-K-ATPase, BSC-1, and TSC in CRF rat kidneys. Am J Physiol. 1999. 277:257–270.
7. Kwon TH, Frokiaer J, Knepper MA, Nielsen S. Reduced AQP1, -2, and -3 levels in kidneys of rats with CRF induced by surgical reduction in renal mass. Am J Physiol. 1998. 275:724–741.
8. Eliahou H, Avinoach I, Shahmurov M, Ben David A, Shahar C, Matas Z, Zimlichman R. Renoprotective effect of angiotensin II receptor antagonists in experimental chronic renal failure. Am J Nephrol. 2001. 21:78–83.

9. Uhlenius N, Miettinen A, Vuolteenaho O, Tikkanen I. Renoprotective mechanisms of angiotensin II antagonism in experimental chronic renal failure. Kidney Blood Press Res. 2002. 25:71–79.

10. Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol. 2003. 285:152–165.
11. Kwon TH, Nielsen J, Masilamani S, Hager H, Knepper MA, Frokiaer J, Nielsen S. Regulation of collecting duct AQP3 expression: response to mineralocorticoid. Am J Physiol Renal Physiol. 2002. 283:1403–1421.
12. Nielsen J, Kwon TH, Masilamani S, Beutler K, Hager H, Nielsen S, Knepper MA. Sodium transporter abundance profiling in kidney: effect of spironolactone. Am J Physiol Renal Physiol. 2002. 283:923–933.
13. Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998. 95:14552–14557.

14. Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003. 41:1143–1150.

15. Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol. 1999. 10:Suppl 11. S178–S183.
16. Guo DF, Tardif V, Ghelima K, Chan JS, Ingelfinger JR, Chen X, Chenier I. A novel angiotensin II type 1 receptor-associated protein induces cellular hypertrophy in rat vascular smooth muscle and renal proximal tubular cells. J Biol Chem. 2004. 279:21109–21120.

17. Mazzolai L, Pedrazzini T, Nicoud F, Gabbiani G, Brunner HR, Nussberger J. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensive mice. Hypertension. 2000. 35:985–991.

18. Knepper MA, Nielsen S, Chou CL, DiGiovanni SR. Mechanism of vasopressin action in the renal collecting duct. Semin Nephrol. 1994. 14:302–321.
19. Stephenson JL. Countercurrent transport in the kidney. Annu Rev Biophys Bioeng. 1978. 7:315–339.

20. Kwon TH, Hager H, Nejsum LN, Andersen ML, Frokiaer J, Nielsen S. Physiology and pathophysiology of renal aquaporins. Semin Nephrol. 2001. 21:231–238.

21. Buerkert J, Martin D, Prasad J, Chambless S, Klahr S. Response of deep nephrons and the terminal collecting duct to a reduction in renal mass. Am J Physiol. 1979. 236:454–464.

22. Tannen RL, Regal EM, Dunn MJ, Schrier RW. Vasopressin-resistant hyposthenuria in advanced chronic renal disease. N Engl J Med. 1969. 280:1135–1141.

23. Fine LG, Schlondorff D, Trizna W, Gilbert RM, Bricker NS. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J Clin Invest. 1978. 61:1519–1527.
24. Teitelbaum I, McGuinness S. Vasopressin resistance in chronic renal failure. Evidence for the role of decreased V2 receptor mRNA. J Clin Invest. 1995. 96:378–385.

25. Kwon TH, Nielsen J, Knepper MA, Frokiaer J, Nielsen S. Angiotensin II AT1 receptor blockade decreases vasopressin-induced water reabsorption and AQP2 levels in Nacl-restricted rats. Am J physiol Renal Physiol. 2005. 288:673–683.

26. Hus-Citharel A, Marchetti J, Corvol P, Llorens-Cortes C. Potentiation of [Ca2+]i response to angiotensin III by cAMP in cortical thick ascending limb. Kidney Int. 2002. 61:1996–2005.

27. Klingler C, Ancellin N, Barrault MB, Morel A, Buhler JM, Elalouf JM, Clauser E, Lugnier C, Corman B. Angiotensin II potentiates vasopressin-dependent cAMP accumulation in CHO transfected cells. Mechanisms of cross-talk between AT1A and V2 receptors. Cell Signal. 1998. 10:65–74.

28. Knepper MA, Kim GH, Fernandez-Llama P, Ecelbarger CA. Regulation of thick ascending limb transport by vasopressin. J Am Soc Nephrol. 1999. 10:628–634.

29. Wang W, Li C, Kwon TH, Miller RT, Knepper MA, Frokiaer J, Nielsen S. Reduced expression of renal Na+ transporters in rats with PTH-induced hypercalcemia. Am J Physiol Renal Physiol. 2004. 286:534–545.

30. Elkjaer ML, Kwon TH, Wang W, Nielsen J, Knepper MA, Frokiaer J, Nielsen S. Altered expression of renal NHE3, TSC, BSC-1, and ENaC subunits in potassium-depleted rats. Am J Physiol Renal Physiol. 2002. 283:1376–1388.
31. Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na(+)-K(+)-2Cl-cotransporter, BSC-1. Am J Physiol. 1996. 271:619–628.
32. Landwehr DM, Klose RM, Giebisch G. Renal tubular sodium and water reabsorption in the isotonic sodium chloride-loaded rat. Am J Physiol. 1967. 212:1327–1333.

33. Brooks HL, Sorensen AM, Terris J, Schultheis PJ, Lorenz JN, Shull GE, Knepper MA. Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC null mice using targeted proteomics. J Physiol. 2001. 530:359–366.
34. Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol. 1999. 277:447–453.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download