Abstract
Infradiaphragmatic extralobar pulmonary sequestration is an extremely rare congenital malformation. It is more frequently diagnosed in the antenatal period due to routine ultrasonic examination of the fetus or in the first 6 months of life, though on rare occasions it is discovered incidentally in adults. A 32-yr-old man presenting with epigastric discomfort and fever was referred. Computed tomographic scanning showed that a 16-cm, multiseptated, dumbbell-shaped, huge cystic tumor was located beneath the diaphragm. On the next day, 850 mL of thick yellowish pus was drained by sonography-guided fine needle aspiration for the purpose of infection control and diagnosis, but no microscopic organisms were found in repeated culture studies. Surgical removal of the cyst was performed through thoracoabdominal incision and most of these pathologic lesions were removed but we could not find the feeding arteries or any fistulous tract to surrounding structures. Histopathologic study revealed that it was extralobar pulmonary sequestration and culture study showed that many WBC and necrotic materials were found but there were no microorganisms in the cystic contents. We report the first case of an infected infradiaphragmatic retroperitoneal extralobar sequestration which was administered a staged management and achieved an excellent clinical course.
Infradiaphragmatic extralobar sequestration (ELS) is extremely uncommon. Most ELS are asymptomatic. They rarely have a fistulous communication with the respiratory or gastrointestinal tract, and thus cystic overinflation and infection are unlikely. To our knowledge, this is the first report of infradiaphragmatic retroperitoneal ELS complicated with infection.
A 34-yr-old male who presented with a 3-week history of epigastric discomfort and a 1-week history of fever was referred to our hospital. He had been treated with intravenous antibiotics for 1 week at the other hospital. On admission the patient's body temperature was 37.7℃ and blood laboratory studies suggested an infectious condition (WBC 15,700/µL, ESR ≥120 mm/hr, CRP 63.2 mg/L, amylase 96 IU/L). Chest radiograph showed an elevated left hemidiaphragm and a calcified mass in the left upper abdomen and computed tomographic scanning revealed that a 16-cm, multiseptated, dumbbell-shaped, huge cystic tumor containing dense mural calcifications was located in the medial aspect to the left lobe of the liver and spleen, and in the upper anterior aspect of the left kidney. The mass extended to the pancreatic tail and its apex was abutting the heart (Fig. 1). On the following day, 850 mL of thick yellowish pus was drained by sonography-guided fine needle aspiration for the purpose of infection control and diagnosis, but no microscopic organisms were found in repeated culture studies.
On the 8th day of admission, magnetic resonance imaging showed a much reduced cyst size, and patient's body temperature was normalized but blood laboratory data revealed still infectious condition (WBC 9,700/µL, ESR ≥120 mm/hr, CRP 44.7 mg/L). The clinical diagnosis was of a subphrenic abscess or pancreatic pseudocyst and surgical treatment was needed. On the 10th day, the procedure was performed with the patient in the left semi-lateral position with a small roll placed posterior to elevate the left side. A thoracoabdominal incision was made through the 11th intercostal space and a careful inspection of the pleural and retroperitoneal spaces was performed. No abnormal findings were revealed in the pleural space but a cystic mass with adhesion to the surrounding organs was found in the retroperitoneal space. Calcified materials and thick yellowish pus were drained from the cyst (Fig. 2). Careful dissection of the adhesions between the cyst and the surrounding structures was performed and most of these pathologic lesions were removed but a severe adhesive lesion between the aorta and diaphragm beneath the heart could be not removed. And neither could the feeding arteries be identified. This lesion was revealed as extralobar pulmonary sequestration finally. Histopathologically, the cyst was lined by ciliated pseudostratified columnar epithelium and surfactants secreted by type II pneumocytes were observed by special staining (Fig. 3). In the cystic contents, many WBC and necrotic materials were found but there were no microorganisms in culture study. The patient was discharged 10 days after the operation and the remnant mass had nearly disappeared at a chest radiograph obtained 3 months later.
The etiology of infradiaphragmatic extralobar pulmonary sequestration has been suggested to be caused by the trapping of sequestered tissue within the infradiaphragmatic space just before pleuroperitoneal membrane closure between gestation weeks 5 and 8 (1). Infradiaphragmatic ELS is extremely uncommon with an estimated incidence of 2-5% of pulmonary sequestration, and of 10-15% for ELS below the diaphragm (2). It is most frequently seen in the first 6 months of life, but is found incidentally, though rarely, in older children and adults (3).
Grossly, ELS is usually a single pyramidal to round or oval lesion, ranging from 0.5-15 cm in largest diameter. It is covered by a smooth to finely wrinkled pleura, and may be adherent to adjacent structures if previously infected (3). The arterial supply in 80% of ESL cases comes directly from the thoracic or abdominal aortas; around 15% receive blood from another systemic artery (celiac, renal artery) and 5% from the pulmonary artery, and venous drainage is predominantly into the systemic circulation, though about 25% drain completely or partially via the pulmonary veins (4). In most cases the sequestration is close to the diaphragm in the left suprarenal area, and is associated with other congenital malformations in approximately 50% of cases. Diaphragmatic hernia, pericardial defects, pectus excavatum, and congenital heart disease are the most frequently reported associated anomalies (5).
Most ELS are asymptomatic. However, the lack of communication between an ELS and the tracheobronchial tree results in fluid accumulation in sequestered tissue, which results in a large size and extrinsic compression. They rarely have a fistulous communication with the respiratory or gastrointestinal tract, and thus cystic overinflation and infection are unlikely. To our knowledge, this is the first report of infradiaphragmatic retroperitoneal ELS accompanied with infection, but we were unable to find any fistulous tract to the respiratory or gastrointestinal tract and any infection source. Arterial shunting and congestive heart failure also are uncommon (6). Spontaneous regression has been reported in patients with intralobar or extralobar intrathoracic sequestration (7) and several hypotheses have been suggested to explain spontaneous involution of the sequestered lung. Progressive fibrosis of dysplastic tissue, or thrombosis and fibrosis of the feeding vessel leading to progressive infarction of the abnormal lung would seem to be the most feasible (7). Accordingly, some authors advocate close follow-up with imaging studies.
The treatment of infradiaphragmatic ELS is controversial, however, operative resection is still the mainstay of treatment. Removal allows confirmation of diagnosis and reassures that a neuroblastoma or a cystic adenomatoid malformation is not being left untreated. Resection also eliminates any risk of malignant transformation, which has been reported in other congenital cystic lung lesions (8). In addition, exploration allows the opportunity to address associated anomalies, such as, congenital diaphragmatic hernias.
In summary, we describe the first case of an infected infradiaphragmatic retroperitoneal ELS, which occurred in a 32-yr-old man, and which presented with epigastric discomfort and fever. We administered a staged management of the infected ELS and achieved an excellent clinical course.
Figures and Tables
Fig. 1
Computed tomographic scan of the abdomen with contrast enhancement. A huge calcified cystic mass is located in the retroperitoneal space on the left side.

References
1. Huang CC, Ko SF, Chung MY, Shieh CS, Tiao MM, Lui CC, Ng SH. Infradiaphragmatic pulmonary sequestration combined with cystic adenomatoid malformation: unusual postnatal computed tomographic features. Abdom Imaging. 2004. 29:439–442.

2. Chan Y, Oldfield R, Vogel S, Ferguson S. Pulmonary sequestration presenting as a prenatally detected suprarenal lesion in a neonate. J Pediatr Surg. 2000. 35:1367–1369.

3. Rajendiran S, Kapoor V, Schoedel K. Fine-needle aspiration cytology of intraabdominal extralobar pulmonary sequestration: a case report. Diagn Cytopathol. 2003. 29:24–27.

5. Carrasco R, Castanon M, San Vincente B, Tarrado X, Montaner A, Morales L. Extralobar infradiaphragmatic pulmonary sequestration with a digestive communication. J Thorac Cardiovasc Surg. 2002. 123:188–189.

6. Danielson PD, Sherman NJ. Laparoscopic removal of an abdominal extralobar pulmonary sequestration. J Pediatr Surg. 2001. 36:1653–1655.

7. Garcia-Pena P, Lucaya J, Hendry GM, McAndrew PT, Duran C. Spontaneous involution of pulmonary sequestration in children: a report of two cases and review of the literature. Pediatr Radiol. 1998. 28:266–270.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



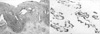
 XML Download
XML Download