Abstract
Gastric CD30-positive anaplastic large-cell lymphoma is a very rare disease. It is sometimes difficult to distinguish it from undifferentiated carcinoma, sarcoma and so on. We report here on a case of primary gastric anaplastic large-cell lymphoma. A 50-yr-old woman complained of epigastric pain and severe chest pain for 1 week. The gastroendoscopic examination revealed geographic mucosal irregularities with shallow ulceration at the antrum. She underwent a total gastrectomy. The gross finding of the resected stomach was an 8×4.5 cm sized ulceroinfiltrative lesion at the pyloric antrum along the lesser curvature. The microscopic examination revealed diffuse and solid proliferations of large atypical cells with pleomorphic nuclei. Immunohistochemically, the tumor cells were positive for CD30, vimentin and CD3, and this was a finding compatible with anaplastic large-cell lymphoma. To the best of our knowledge, this is the first such reported case in Korea.
CD30-positive anaplastic large-cell lymphoma (ALCL) is a rare subtype of non-Hodgkin's lymphoma that was described by Stein et al. in 1985 (1). The lymphoma usually occurs in lymph nodes, but the disease is known to involve various extranodal sites such as the skin (the most common site), and bone, lung, spleen and the gastrointestinal tract (2). In Korea, Cho et al. (3) have reported on multiorgan involvement of ALCL that included the stomach.
Primary gastric CD30-positive ALCLs are very rare, and there have been only a few such cases reported worldwide. Distinguishing gastric ALCLs from other the carcinomas or sarcomas may be confusing (2, 4, 5). Thus, a careful histologic examination for such cases is needed. We report here on a case of gastric Ki-1-positive ALCL that was proven pathologically. To the best of our knowledge, this is the first reported case of primary gastric ALCL in Korea.
In March 2004, a 50-yr-old woman was admitted to our hospital complaining of epigastric pain and severe anterior chest pain of 1 week duration. She was without any significant prior medical history. An electrocardiogram and an echocardiogram showed no significant abnormalities of the heart. We concluded that the chest pain was atypical and that it did not originate from the heart. Gastroendoscopic examination revealed a geographic mucosal irregularity at the antrum (Fig. 1A), and we performed a biopsy at the site. The pathology examination showed undifferentiated anaplastic neoplasm. Endoscopic ultrasonography showed the mucosal thickening (Fig. 1B), and the computerized tomography scan of the abdomen showed no evidence of distant metastasis. We performed follow-up gastroendoscopic examination after 2 weeks. The lesion was then more aggravated; her pain was persistent and did not respond to H2-blocker or proton pump inhibitors. She underwent total gastrectomy and the dissection of 16 regional lymph nodes.
The gross finding of the resected stomach tissue revealed an 8×4.5 cm well-demarcated ulceroinfiltrative lesion at the pyloric antrum along the lesser curvature (Fig. 2), which infiltrated into the submucosal layer. There were many enlarged lymph nodes. The microscopic examination of the resected stomach tissue revealed a diffuse and solid proliferation of large atypical cells with pleomorphic nuclei. Some giant tumor cells were confined in the mucosa-submucosa area, and they showed multiple nuclear lobulations (Fig. 3A).
An immunohistochemical study was performed on the formalin-fixed, paraffin-embedded surgical specimens by the streptavidin-biotin-peroxidase complex method (LSAB kit; DAKO, Japan). The tumor cells were positive for CD30 (Fig. 3B), vimentin and CD3, but they were negative for cytokeratin, carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), CD45, CD20, CD45RO and anaplastic lymphoma kinase (ALK) (Fig. 3C). Eight of the sixteen lymph nodes contained metastasized tumor.
The patient had no B symptoms, and there was no bone marrow involvement. She had no palpable peripheral lymph nodes and there were no abnormal findings on chest radiography. The initial laboratory test results were as follows: white blood cell count, 4,300/µL; hemoglobin level, 10.3 g/dL; platelet count, 196,000/µL; lactate dehydrogenase level, 716 IU/L (240-460 IU/L). The serum calcium level, aspartate aminotransferase level and alanine aminotransferase level were normal. Her ECOG performance status was 1.
We diagnosed her as having CD30-positive primary gastric ALCL IIE, and we started chemotherapy with cyclophosphamide, adriamycin, vincristine and prednisolone. After two cycles of chemotherapy, prolonged leukopenia was noted. We then performed a bone marrow examination. The bone marrow revealed a hypocellular marrow (the cellularity was 10%) without evidence of tumor involvements. We decided to stop any further chemotherapy and the patient was closely followed up. At 7 months after diagnosis, the patient remains alive and without tumor recurrence.
The stomach is a common site of extranodal lymphoma occurrence, and most of the gastric lymphomas are of the B-cell type. Primary gastric CD30-positive ALCLs are very rare. There have been several previous reports regarding primary gastric ALCL in Japan and China (6-9); however, to the best of our knowledge, this is the first such reported case in Korea. We can only guess that it is indeed a very rare disease.
The ALCL cells are large and they have pleomorphic or multiple nuclei. Thus, primary gastric ALCL is sometimes misdiagnosed as undifferentiated carcinoma, melanoma, histiocytic neoplasm, sarcoma and so on (2, 4, 5). Unfortunately, we could not initially confirm the type of malignancy. The tumor cells of ALCL are CD30 positive and usually express EMA and one or more T-cell-associated antigens (1, 10). CD30 is a transmembrane cytokine receptor of the tumor necrosis factor receptor family. In the pathologic condition, it is expressed on the Reed-Sternberg cells of Hodgkin lymphoma and ALCL. CD30 is also observed in many categories of lymphoid tumors other than ALCL, and also can be expressed on embryonal carcinoma, however, the positivity of CD30 is usually weak in these cases. Other non-lymphoid malignant cells lack this CD30 expression. Although expression of CD30 is the hallmark of ALCL, it is not specific just for this disease. Therefore, it is necessary to perform other immunohistochemistry tests for other lymphoid and non-lymphoid markers (11). In our case, the tumor cells showed a similar morphology to those of ALCL. Immunohistochemically, most of the tumor cells were positive for CD30 and CD3, but negative for cytokeratin, CEA, EMA, thus confirming ALCL.
Anaplastic lymphoma kinase (ALK) expression is found in 50-70% of CD30-positive ALCL, and 75% of these cases have chromosomal abnormalities. ALCL is divided into three subgroups according to the ALK expression: ALK-positive systemic ALCL, ALK-negative systemic ALCL, and primary cutaneous ALCL (ALK negative). ALK-positive ALCL usually occurs at a younger age (15-30 yr), it is a male predominant disease, it exhibits frequent B-symptoms, it is known to be more chemosensitive and to have a better prognosis for survival (14, 15). However, Sachiko et al. have reported that one of three patients with gastric CD30-positive ALCL was positive for ALK and EMA (6). In our case, the tumor cells were negative for ALK immunohistochemistry, and the clinical characteristics of our case were in accord with the usual clinical findings for ALK-negative systemic ALCL.
Gastric T/null-cell lymphoma may be associated with Helicobacter pylori infection. Bariol et al. have reported gastric T-cell lymphoma regression with the eradication of H. pylori (9). In the present case, although we could not find H. pylori in the biopsy specimen, the urea breath test was positive. We performed H. pylori eradication with amoxicillin, clarythromycin and rabeprazole before obtaining the pathology results. However, the lesion became aggravated. Further study on the association between gastric T-cell lymphoma and H. pylori infection is needed.
Little is known about the treatment and prognosis of gastric ALCL. Most cases of gastric ALCL have been treated with surgical resection, and some patients have received chemotherapy after surgery. A few patients have received chemotherapy alone (12). The outcomes of treatment have been variable despite the different methods of treatment. Cases of primary gastric ALCL are very rare, so a standard treatment is not known. T-cell ALCL is generally an aggressive lymphoma, and Nakamura et al. have reported on rapidly progressive gastric ALCL (8). The treatment regimens for primary systemic ALCL are the same as those used for diffuse large B-cell lymphoma (13). Therefore, we performed chemotherapy with CHOP (cyclophosphamide, doxorubicin hydrochloride, oncovin [vincristine], and prednisolone) after the patient's total gastrectomy. After two cycles of chemotherapy, we were forced to stop the treatment because of the poor bone marrow reservoir. At 5 months after diagnosis, the patient is alive and there has been no tumor recurrence.
We can suggest that ALCL should be considered among the cases of pleomorphic tumors of the stomach.
Figures and Tables
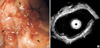 | Fig. 1Gastroendoscopy shows a geographic mucosal irregularity at the antrum (A). Endoscopic ultrasonography shows submucosal thickening of the stomach (arrows) (B). |
 | Fig. 2A macroscopic picture of the total gastrectomy specimen. The ulceroinfiltrative mass measuring 8×4.5 cm can be seen on the lesser curvature of the antrum. |
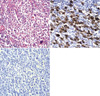 | Fig. 3Histologic and immunohistochemical pictures of the gastric tumor. A high power view (H&E, ×400) shows a diffuse and solid proliferation of large atypical cells with pleomorphic nuclei. Multiple nuclear lobulations are noted (A). Positive immunoreactivity with anti-CD30 (×400) and (B), negative immunoreactivity for ALK (×400) (C). |
ACKNOWLEDGMENT
We thank all the members of Department of Pathology, Seoul National University College of Medicine, for their technical assistance.
References
1. Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, Schwarting R, Lennert K. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985. 66:848–858.

2. Ross WR, Hanson CA, Schnitzer B. CD30 (Ki-1)-positive, anaplastic large cell lymphoma mimicking gastrointestinal carcinoma. Cancer. 1992. 70:2517–2523.

3. Cho GS, Cho ET, Moon CH, Park YK, Lee MJ, Park YH, Chung CH, Jeon Ho Jong. A case of Ki-1 positive anaplastic large cell lymphoma metastasis to the stomach, pleura and cecum. Korean J Hematol. 1995. 30:501–507.
4. Falini B, Pileri S, Stein H, Dieneman D, Dallenbach F, Delsol G, Minelli O, Poggi S, Martelli MF, Pallesen G. Variable expression of leukocyte-common (CD45) antigen in CD30 (Ki-1)-positive anaplastic large-cell lymphoma: implications for the differential diagnosis between lymphoid and nonlymphoid malignancies. Hum Pathol. 1990. 21:624–629.
5. Lee JW, Oh YL, Ko YH. Fine needle aspiration cytology of anaplastic large cell lymphoma: a mimicking malignant fibrous histiocytoma. Korean J Cytopathol. 1998. 9:99–104.
6. Iwamizu-Watanabe S, Yamashita Y, Yatabe Y, Nakamura S, Mori N. Frequent expression of CD30 antigen in the primary gastric non-B, non-Hodg- kin lymphomas. Pathol Int. 2004. 54:503–509.
7. Mori N, Yatabe Y, Oka K, Yokose T, Ishido T, Kikuchi M, Asai J. Primary gastric Ki-1 positive anaplastic large cell lymphoma: a report of two cases. Pathol Int. 1994. 44:164–169.

8. Nakamura S, Aoyagi K, Ohkuni A, Kimura Y, Tsuneyoshi M, Fujishima M. Rapidly growing primary gastric CD30 (Ki-1)-positive anaplastic large cell lymphoma. Dig Dis Sci. 1998. 43:300–305.
9. Bariol C, Field A, Vickers CR, Ward R. Regression of gastric T cell lymphoma with eradication of Helicobacter pylori. Gut. 2001. 48:269–271.

10. Chou WC, Su IJ, Tien HF, Liang DC, Wang CH, Chang YC, Cheng AL. Clinicopathologic, cytogenetic, and molecular studies of 13 Chinese patients with Ki-1 anaplastic large cell lymphoma. Special emphasis on tumor response to 13-cis retinoic acid. Cancer. 1996. 78:1805–1812.
11. Falini B, Pileri S, Pizzolo G, Dürkop H, Flenghi L, Stirpe F, Martelli MF, Stein H. CD30 (Ki-1) molecule: A new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995. 85:1–14.

12. Paulli M, Rosso R, Kindl S, Boveri E, bonoldi E, Stracca V, Motta T, Arrigoni G, Lazzarino M, Menestrina F, Magrini U. Primary gastric CD30 (Ki-1)-positive large cell non-Hodgkin's lymphomas: A clinicopathologic analysis of six cases. Cancer. 1994. 73:541–549.

13. Armitage JO, Mauch PM, Harris NL, Bierman P. DeVita VT, Hellman S, Rosenberg SA, editors. Lymphomas. Cancer: Principles and Practice of Oncology. 2001. 6th ed. Philadelphia: Lippincott;2297–2298.
14. Greer JP. Greer JP, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B, editors. Non-Hodgkin lymphomas in adults. Wintrobe's Clinical Hematology. 2004. Vol 2:2nd ed. Philadelphia: Lippincott;2383–2384.
15. Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, Pileri S, Falini B. CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000. 96:3681–3695.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download