Abstract
Human skin color shows variations throughout life and influenced by various factors such as race, sex, age and hormones. Since the development of spectrophotometer, many studies on human skin color have been done. However, few studies have been carried out to measure the skin color of neonatal infants. The aim of our study was to assess the variations in skin color according to site, gestational age, birth weight and season of birth in Korean neonates. A total of 447 healthy neonates (3 days after birth, 213 males and 234 females) were enrolled in the present study. Skin pigmentation was measured by reflectance spectrophotometer (Derma-Spectrophotometer®, Cortex technology, Hadsund, Denmark) at four different sites (forehead, upper arm, abdomen, and inguinal area). The forehead showed highest melanin index in all sites measured (p<0.05). There was no significant difference according to gestational age, birth weight, and season of birth. This result imply that the skin color in neonates is mainly determined genetically.
Normal human skin color can be classified either as constitutive pigmentation or facultative pigmentation (1). Constitutive skin color is defined as the basal or genetically determined color in the absence of any external factor such as sunlight. Facultative skin color is that which develops following exposure to a stimulant such as sunlight. There are many factors that determine the color of human skin. They include quantity of melanin pigment and its chemical structure and nonmelanin pigments such as oxygenated and reduced hemoglobin, carotene, and other chemicals like lycopenic acid or licorice (2). Many chemicals in foods or food supplements and medication can alter the color of skin. Various factors including race, sex, age and hormones are reported to have an influence on change of human skin color (3-8).
Several methods are available for assessing skin colors. Recently, a hand-held microprocessor controlled reflectance spectrophotometer (Derma-Spectrophotometer®, Cortex technology, Hadsund, Denmark) began to be used in many dermatological studies. This instrument provides a readout of the erythema and melanin indices as a function of the absorbance characteristic of human skin (9). Each index increases as the skin becomes more erythematous and more pigmented, respectively, so the melanin index (M-index) can be regarded as a parameter which is mainly influenced by the melanin content (10).
The main purpose of this study is to investigate the variations of skin color according to site, gestational age, birth weight and season of birth in Korean neonates by using a reflectance spectrophotometer (Derma-Spectrophotometer®, Cortex technology, Hadsund, Denmark).
A total of 447 healthy neonates (3 days after birth, 213 males and 234 females) were enrolled in the present study. All of neonates had a known gestational age (35-42 weeks) calculated from the date of mothers last menstrual period. Birth weight was calculated from same body weight scale. To assess variation of season of birth, they were classified into as follows; spring (between March and May, n=140), summer (between June and August, n=101), autumn (between September and November, n=96), winter (between December and February, n=110). Neonates with conditions causing pigmentation and those with pigmentary skin diseases were excluded.
Skin pigmentation was measured by reflectance spectrophotometer (Derma-Spectrophotometer®, Cortex technology, Hadsund, Denmark) at four sites (forehead, midway between the hair-line and the base of nose, medial aspect of the upper arm approximately midway between the scapular-humeral and ulnar-humeral joints, abdomen just above the umbilicus, and inguinal area, midway between external genital and inguinal fold) after calibration to every series of the measurement. On every subject, three readings were taken at each of four sites. All skin color examinations were made in the same room circumstances that exposure to sunlight was almost completely obviated and room temperature maintained between 21℃ and 26℃.
The reflectance spectrophotometer is a narrow-band spectrophotometer designed for measuring specific colors due to two major chromophores, hemoglobin and melanin. The light sources are two light-emitting diodes with selected narrow bands of emitted wavelengths. The peaks of the two bands are centered at 568 nm (green light) and 655 nm (red light). They emit light in sequence, and the reflected light from skin is detected with a photodetector. After being converted into digital form with a built-in microcomputer, the reflectance in the two bands are transformed into the erythema index (E-index) and the melanin index (M-index).
All measurements of M-index were expressed by mean±SD. The evaluation of gender differences was performed using the unpaired t-test. Differences in skin pigmentation according to body sites, gestational age, birth weight and season of birth were analysed by Turkey's multiple comparison. We considered p<0.05 to be significant.
No significant difference of M-index according to sex was observed in all sites measured (Fig. 1). However, male showed slightly higher M-index than female, except inguinal area, but the statistical significans was not observed. The forehead showed the highest M-index in all sites measured (p<0.05). The inguinal area showed higher M-index than that of the upper arm and the abdomen (p<0.05). There was no difference of M-index between the upper arm and the abdomen.
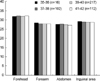
To assess variation of gestational age, they were classified into as follows; GA 35-36 weeks (n=16), 37-38 weeks (n=102), 39-40 weeks (n=217), 41-42 weeks (n=112). There was no significant difference of M-index according to gestational age in all sites measured (Fig. 2).
To assess variation of birth weight, they were classified into as follows; 2.00-2.49 kg (n=12), 2.50-2.99 kg (n=79), 3.00-3.49 kg (n=225), 3.50-3.99 kg (n=108), 4.00-4.49 kg (n=23). In all sites measured, no significant difference of M-index according to birth weight was observed (Fig. 3).
In all sites measured, no significant difference of M-index according to season of birth was observed (Fig. 4).
It is generally thought skin color is fairly constant throughout the life of an individual. In reality, however, the color of the skin shows considerable variation throughout the life of an individual (3-8). There are many intrinsic and extrinsic factors that determine the color of human skin. For a long time, variation of human skin color has been of great concern for physical anthropologist. However, visual comparisons of skin color with standardized sets of colored paper were too subjective to obtain reliable results (11). Since the development of spectrophotometer, many studies on human skin color have been done. However, few studies have been carried out to measure the skin color of neonatal infants (5, 12-14).
According to several studies, it has been observed that females are significantly darker than males just prior to the onset of menarche (1, 15, 16). Except this time, females are reported to be lighter than males (1, 17, 18). Gender differences after the onset of puberty may be associated with the effect of sex hormones. In neonates, it has been reported that males consistently had a slightly darker skin than females although the differences were not statistically significant (12). In our study, males showed slightly higher level of M-index except inguinal area, but the statistical significance was not observed (Fig. 1).
Our data that pigmentation in neonates was at its highest level at the forehead is similar to previous report. According to Post et al. (12), the forehead of both black and white term infants measured within hours after birth was average 10-12% darker, respectively than the upper arm or the sternum. Lim and Lee (5) also reported that forehead showed relatively darker pigmentation than the forearm and abdomen in all age groups including neonates. Site difference is probably due to a difference in the density of epidermal melanocytes in the forehead compared with other sites. According to several reports, it has been observed that genitalia have relatively higher density of melanocytes populations than does the face. Our research, however, indicates the opposite to be the report. In our study, the reason that inguinal area showed lower levels of M-index than the forehead was because measurement was taken from between the genitalia and the inguinal folds, rather than the genitalia itself.
A number of skin reflectance studies have documented age change at various stages in the life cycle (1, 5, 6, 16). Several clinical studies have shown skin color progressively darkens from infancy to the onset of puberty, and then continue to lighten after adulthood. It has been estimated that the population density of melanocytes decreases about 10% per decade after age 25-30 yr (19). Walsh (13) reported newborn infants to be much lighter skinned than adults, but to reach young adult values by six months of age. In the present study, neonates between gestational age 35 weeks and 42 weeks showed no significant difference according to gestational age in all sites measured (Fig. 2). Our data also showed there was no significant difference of M-index according to birth weight (Fig. 3).
Lock-Anderson and Wulf (4) reported that there was considerable seasonal variation for skin pigmentation at the UV-exposed sites. Also, they showed that covered buttock skin had a pigmentation and UV sensitivity that varied only marginally. An endogenous circulating melanocytic factor originating from the irradiated skin has been suggested to account for the melanocyte increase in covered skin being able to activate silent melanocytes or stimulated melanocyte proliferation. We have not found significant differences of M-index according to the season of birth in neonates (Fig. 4). This result suggests that the circulating melanocytic factors such as α-MSH of the mother have no effect on the skin color of neonates.
Figures and Tables
References
1. Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne JP. Nordlund JJ, Ortonne JP, editors. The pigmentary system. The normal color of human skin. 1998. 1st ed. New York: Oxford University Press;475–478.

2. Anttila VJ, Makela JP, Soininen K, Helminen V, Tenhunen R. Discolouration of skin and serum after sweet ingestion. Lancet. 1993. 5:1476–1477.

4. Lock-Andersen J, Wulf HC. Seasonal variation of skin pigmentation. Acta Derm Venereol (Stockh). 1997. 77:219–221.
5. Lim TW, Lee MH. A study of skin color by melanin index according to sex, age, site and skin phototype in Koreans. Ann Dermatol. 2002. 14:71–76.

6. Roh KY, Kim D, Ha SJ, Ro YJ, Kim JW, Lee HJ. Pigmentation in Koreans: study of the differences from Caucasians in age, gender and seasonal variations. Br J Dermatol. 2001. 144:94–99.

9. Troilius A, Ljunggren B. Reflectance spectrophotometry in the objective assessment of dye laser treated port-wine stains. Br J Dermatol. 1995. 132:245–250.
10. Takiwaki H, Overgaard L, Serup J. Comparison of narrow-band reflectance spectrophotometric and tristimulus colorimetric measurements of skin color. Skin Pharmacol. 1994. 7:217–225.

11. Harrison GA. Differences in human pigmentation: measurement, geographic variation, and causes. J Invest Dermatol. 1973. 60:418–426.

12. Post PW, Krauss AN, Walcman S, Auld PA. Skin reflectance of newborn infants from 25 to 44 weeks gestational age. Hum Biol. 1976. 48:541–557.
13. Walsh RJ. Variation of the melanin content of the skin of New Guinea natives at different ages. J Invest Dermatol. 1964. 42:261–265.
14. Grande R, Gutierrez E, Latorre E, Arguelles F. Physiological variations in the pigmentation of newborn infants. Hum Biol. 1994. 66:495–507.
15. Frost P. Human skin color: a possible relationship between its sexual dimorphism and its social perception. Perspect Biol Med. 1998. 32:38–58.

16. Kahlon DP. Age variation in skin color: a study in Sikh immigrants in Britain. Hum Biol. 1976. 48:419–428.
17. Clark P, Stark AE, Walsh RJ, Jardine R, Martin NG. A twin study of skin reflectance. Ann Hum Biol. 1981. 8:529–541.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download