Abstract
Tumor necrosis factor-Related Apoptosis-Inducing Ligand (TRAIL) has been reported to specifically kill malignant cells but to be relatively nontoxic to normal cells. One of disadvantages to previous in vivo protocols was the need for large quantities of TRAIL recombinant protein to suppress tumor growth. To evaluate the antitumor activity and therapeutic value of the TRAIL gene, we constructed adenoviral vectors expressing the human TRAIL gene (Ad.hTRAIL) and transferred them into malignant glioma cells in vitro and tumors in vivo, as an alternative to recombinant soluble TRAIL protein. The results show that TRAIL-sensitive glioma cells infected Ad.hTRAIL undergo apoptosis through the production and expression of TRAIL protein. The in vitro transfer elicited apoptosis, as demonstrated by the quantification of viable or apoptotic cells and by the analysis of cleavage of poly (ADP-ribose) polymerase. Furthermore, in vivo administration of Ad.hTRAIL at the site of tumor implantation suppressed the outgrowth of human glioma xenografts in SCID mice. These results further define Ad.hTRAIL as an anti-tumor therapeutic and demonstrate its potential use as an alternative approach to treatment for malignant glioma.
Apo2L/TRAIL (Apo2 ligand or tumor necrosis factor (TNF)-related apoptosis-inducing ligand) was discovered by its sequence homology to TNF and CD95L (1, 2). Recombinant soluble human Apo2L/TRAIL is a candidate for clinical investigation in cancer therapy because it induces apoptosis in a broad spectrum of human cancer cell lines but not in many normal cells, and exhibits potent anti-tumor activity without normal tissue toxicity in various cancer xenograft models (3-7). To date, four homologous, but distinct, human TRAIL (hTRAIL) receptors have been identified, with two (DR4/TR1 [8] and DR5/TR2 [9-11]) having the ability to initiate the apoptosis signaling cascade after ligation, and two (TRID/DcR1/TR3 [9, 11, 12] and TRUNDD/DcR2/TR4 [13-15]) lacking this ability. Because they lack the ability to directly signal cell death, TR3 and TR4 have been hypothesized as being protective receptors, either by acting as decoy receptors (8, 14, 15) or via transduction of an antiapoptotic signal (13).
Glioblastoma multiforme (GBM) is the most malignant and common brain tumor, comprising 23% of all primary brain tumors in adults. GBM tumors are refractory to all current therapeutic approaches, including surgery, radiotherapy, and chemotherapy (16). Human glioma cells express TR2 and undergo apoptosis upon TRAIL ligation in vitro (17, 18). Local injection of TRAIL exerted strong antitumor activity on intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity (6). In combination with conventional DNA-damaging chemotherapy, TRAIL showed synergistic cytotoxicity for human gliomas in vivo and in vitro (19). Therefore, systemic or intracranial TRAIL treatment may be a promising approach for human GBM tumors. However, one potential drawback to these findings was that large amounts of soluble TRAIL were required to inhibit tumor formation. This may be due to the pharmacokinetic profile of soluble TRAIL that indicated that after intravenous injection the majority of the protein is cleared within 5 hr (4). Increasing the in vivo t1/2 of soluble rTRAIL or developing an alternative means of delivery may increase the relative tumoricidal activity of TRAIL such that larger, more established tumors could be eradicated as efficiently as smaller tumors. The results presented in this work describe the production of an adenoviral vector engineered to carry the gene for hTRAIL. After infection, TRAIL protein was detected, leading to the induction of apoptosis in human malignant glioblastoma cells in vitro as well as significant antitumor activity in vivo. These results demonstrate the potential therapeutic utility of adenoviral-mediated delivery of TRAIL gene.
The cDNA for hTRAIL was obtained from Dr. Hideo Yagita (Juntendo University, Tokyo, Japan) (20). To generate E1-deleted recombinant adenoviral vector encoding hTRAIL (Ad.hTRAIL), hTRAIL cDNA was introduced into the shuttle plasmid, pAvCvSv, under the transcriptional control of the cytomegalovirus (CMV) immediate early enhancer/promoter (21). The recombinant shuttle plasmid was co-transfected with the E1-deleted adenovirus serotype 5 genome, pJM17 (22), into transformed human embryonic kidney cells, designated 293 cells (23). A E1-deleted recombinant adenovirus, containing a reporter β-galactosidase (β-Gal) gene with CMV promoter (Ad.lacZ) was used for concurrent control. Recombinant adenoviruses were amplified on 293 cells and purified by two centrifugation steps on cesium chloride gradients (24). Viruses were dialyzed against 10 mM Tris HCl pH 8.0, 1 mM MgCl2, 10% glycerol and stored at -80℃ until use. The number of viral particles was assessed by measurement of the optical density at 260 nm.
The p53-wild-type human malignant glioma cell lines U87MG and the p53-mutated U373MG were obtained from the American Type Culture Collection. Each of these cell lines was maintained in growth medium consisting of RPMI1640 supplemented with 10% fetal celf serum (Life Technologies, Calsbad, CA, U.S.A.), and the following anti-microbial agents: 100 IU/mL penicillin and 100 µg/mL streptomycin. Cultures were maintained at 37℃ in a humidified atmosphere with 5% CO2 in air and subcultured every 4 to 7 days with 0.25% trypsin in HBSS (Life Technologies).
Cells were cultured and permitted to adhere for at least 12 hr before adding adenovirus. Before infection, cells were washed with PBS, and then the vectors were added at the indicated number of multiplicity of infection (MOI)/cell in PBS. After 30 min, cells were washed with PBS and incubated in complete medium for the remainder of the assay.
Cells were cultured and harvested on second and third day after infection of Ad.hTRAIL and treated with lysis buffer (0.5% sod. deoxycholate, 0.5% Triton X-100, 50 mM Tris, pH 7.4, 150 mM NaCl, 62.5 mM sucrose, 5 mM EDTA, 1 mM PMSF). Protein concentrations of the lysates were determined by the colorimetric bicinchoninic acid analysis (Pierce, Rockford, IL, U.S.A.). Equal amounts of protein were separated by SDS-PAGE, transferred to nitrocellulose membrane (Novex, San Diego, CA, U.S.A.). The membrane was incubated with the anti-PARP (BD Biosciences, San Jose, CA, U.S.A.), or anti-hTRAIL mAb (BD Biosciences). For detection of secreted TRAIL, the medium of the TRAIL-expressing cell cultures were measured by ELISA (BD Biosciences).
Cells were cytocentrifuged, stained in 4 µg/mL Hoechst 33342 for 30 min at 37℃ and fixed for 10 min in 4% paraformaldehyde.
Cells were infected with 10, 30, 100, 300, and 1,000 MOI of Ad.hTRAIL; 10% (volume/volume) of stock MTT solution (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (Sigma, St. Louis, MO, U.S.A.) dissolved in medium at a concentration of 5 mg/mL was added to the medium directly, and cells were incubated for 30 min at 37℃, to allow cell-mediated reduction of MTT, to assay cell viability. The medium was aspirated and cells were washed with phosphate-buffered saline. DMSO (0.5 mL, equal to the volume of medium) was added to solublize the dye. Absorbance was measured at 570 nm.
In vivo assessment of the effect of Ad.hTRAIL on glioma growth was performed using a SCID mice (6-8 weeks old; Daehan Laboratory Animal Center, Korea) by subcutaneous inoculation of 1×106 U87MG glioma cells into the dorsal flank of each mouse. This dose of cells produces virtually 100% tumor take in the SCID mouse system in our laboratory. To examine the apoptotic action of Ad.hTRAIL, 10 days after tumor implantation, animals were randomly chosen to receive either vehicle or 1×1010 particles of Ad.hTRAIL per mouse administered by intratumoral injection a volume of 100 µL. After 3 days the mice were sacrificed and the tumor region was excised. Histological analysis was followed. Furthermore, to determine the suppression of tumor growth of Ad.hTRAIL, animals were administered with Ad.hTRAIL or Ad.lacZ. Tumor volumes were measured every other day for 30 days.
The excised tumor tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Five µm thick sections were stained with hematoxylin-eosin for histological examination. Apoptosis induced in tumor tissues was measured using the TUNEL assay (Apoptosis Detection Kit, Chemicon, Temecula, CA, U.S.A.). Tissue sections, 5 µm thick, were deparaffinized and rehydrated through a series of graded alcohols. The sections were processed in 0.05 M sodium citrate buffer (pH 6.0) and heated in a microwave for 10 min for antigene retrieval. Samples were subjected to the reaction with terminal deoxynucleotidyl transferase in the presence of digoxigenin-conjugated nucleotide substrate at 37℃ for 30 min. After the reaction was stopped, the slides were incubated with anti-digoxigenin antibody that had been conjugated with peroxidase. The peroxidase was visualized with DAB (3,3'-diaminobenzidine tetrahydrochloride). Sections were counterstained with Mayer's hematoxylin and then coverslipped.
The cDNA encoding full-length hTRAIL was inserted into the E1 region of a replication-deficient Ad5 construct under the control of the CMV immediate early promoter. The engineered TRAIL-expressing adenovirus was infected into U87MG and U373MG cells, and the cellular proteins were separated by SDS-PAGE to assay for TRAIL expression by Western blotting. Amino acid sequence analysis of the TRAIL cDNA predicts a weight of 32.5 kDa for TRAIL monomers (1, 2). Treatment of cells with Ad.hTRAIL resulted in a strong TRAIL-specific band according to particle number of infection and period for incubation (Fig. 1). To confirm the secretion of TRAIL in the medium of the TRAIL-expressing U87MG cell cultures, ELISA showed that the concentration of TRAIL is 235±28 pg/mL.
To test whether treatment with the Ad.hTRAIL, which is known to elicit apoptosis in a variety of transformed or malignant cells (3, 4), would similarly elicit apoptosis in cultured cancer cells, the cell-killing effect of the Ad.hTRAIL was analyzed by quantifying apoptotic cells by measuring cell viability via MTT assay (Fig. 2). The results show a significant difference in cell killing between lines in response to treatment with TRAIL-expressing vectors versus control vectors. Although MTT assay of the tumor cells infected with Ad.hTRAIL as presented in Fig. 2 indicates the amount of cell death, it does not discriminate between apoptotic and necrotic cell death. Previous reports have demonstrated that TRAIL-induced cell death occurs through an apoptotic mechanism characterized by the activation of a cascade of intracellular proteases and the cleavage of numerous intracellular proteins (25-27). To confirm that the tumor cell death following Ad.hTRAIL infection was mediated through an apoptotic mechanism, nuclear fragmentation and cellular protein cleavage were examined. Morphological studies using Hoechst nuclear staining revealed nuclear condensation in cells treated with Ad.hTRAIL (Fig. 3A). U87MG and U373MG cells were incubated with Ad.hTRAIL for 30 min, cell lysates were prepared at various times after infection, and the cellular proteins were separated by SDS-PAGE for Western blot analysis of PARP cleavage as substrate for caspase-3 (Fig. 3B). These results demonstrated that treatment with the TRAIL gene effectively elicited apoptosis in cultured malignant glioma cells.
To further test the antitumor effect of the TRAIL gene, human malignant glioma xenografts were established in SCID mice by inoculating U87MG cells subcutaneously into the dorsal flanks of mice as described above in "Materials and Methods." Histochemical analysis by H&E 3 days after intratumoral injection of Ad.hTRAIL into preexisting tumor revealed extensive disappearance of tumor cells in center which demonstrated that intralesional administration of the TRAIL gene effectively killed tumor cells (Fig. 4B). Ad.lacZ-infected tissue showed well preserved and proliferated tumor cells (Fig. 4A). Furthermore, TUNEL assay demonstrated that the Ad.hTRAIL-infected cells showed positive stain for apoptotic cells at periphery (Fig. 4D). while Ad.lacZ-treated cells were not stained (Fig. 4C). To demonstrate whether Ad.hTRAIL was able to suppress growth of preexisting tumor, we gave a single intratumoral injection of Ad.hTRAIL or Ad.lacZ at 7 days after tumor implantation. Ad.hTRAIL significantly suppressed tumor growth (p<0.01) (Fig. 5). The growth of the tumors treated with Ad.hTRAIL was well suppressed until day 30 on average.
Human malignant glioma cells are highly resistant to multiple pro-apoptotic stimuli, including irradiation and cytotoxic drugs. Although loss of p53 function and alterations in cell cycle control genes have been attributed a role in this radiochemoresistant phenotype, the precise molecular pathways mediating glioma cell resistance to radiotherapy or chemotherapy have not been elucidated. Therefore, death ligand, TRAIL is potentially valuable agent for the induction of glioma cell death (17, 28). TRAIL has become an attractive molecule for the treatment of cancers because it specifically kills tumor cells (3, 4). Recently it is reported that TRAIL induced apoptosis through death receptor 5 (DR5) and was mediated by caspase-8-initiated extrinsic and intrinsic mitochondrial pathways in sensitive glioma cell lines. Furthermore, TRAIL alone or in combination with chemotherapeutic agents, induced apoptosis in primary tumor cultures from patients with malignant gliomas, reinforcing the potential of TRAIL as an effective therapeutic agent for malignant gliomas (33). The tumor-specific activity of TRAIL was extended in vivo with the observation that treatment of SCID and nude mice bearing human tumors with soluble TRAIL significantly inhibited tumor outgrowth without any observable toxic side effects to the host (5-7). This inhibition of tumor outgrowth, though, required high amounts of recombinant TRAIL given over several days shortly after tumor implantation. Pharmacokinetic analysis revealed that soluble TRAIL given to mice intravenously displayed an elimination t1/2 of just under 5 hr (4). An alternative approach would be to administer TRAIL locally, where it would exist at a greater concentration and have a better chance of significantly inducing tumor cell death. Such localized, intratumoral injections of soluble TRAIL would, however, be limited in that only a relatively small volume could be administered, suggesting that a potentially suboptimal amount of TRAIL protein would be used. In contrast, Ad.hTRAIL can be produced at high titers, such that small volumes would contain high numbers of infectious adenoviral particles carrying the hTRAIL gene.
In the study reported here, we asked whether introducing the TRAIL gene via adenoviral vector directly into malignant glioma cells would result in the expression of biologically active molecules that could effectively kill malignant cells in vitro and in vivo. The data presented in this study describe the generation of an adenoviral vector engineered to carry the gene for hTRAIL. Ad.hTRAIL infection resulted in the transcription and translation of the transferred hTRAIL gene into functional TRAIL protein that, when expressed on the cell surface, induced apoptotic death in malignant glioma cells. One report described that adenoviral TRAIL gene transfer is ineffective treatment strategy for malignant glioma (29). In this study, however, adenoviral TRAIL gene transfer showed apoptosis in human glioma cells and even induced cell death in TRAIL-resistant glioma cells (U373MG). We have found that treatment with TRAIL-expressing vectors produced a substantial amount of TRAIL on the surface of the cells and secreted soluble TRAIL in the medium of the TRAIL-expressing cell cultures. These results suggest that the apoptotic activity of TRAIL produced by adenoviral vector is elicited via both of membrane-bound TRAIL and soluble TRAIL. The effects of soluble TRAIL may be dose-dependent, conformation-dependent, or both. It has been reported that trimerized recombinant TRAIL is more effective than the monomer form; thus, conformational differences in soluble TRAIL proteins may also dramatically affect their activities (30). Our data suggest that transmembrane expression is indeed a highly potent strategy for expressing TRAIL. Why transmembrane expression of TRAIL is so highly efficient for inducing target cell apoptosis is unclear, although possibilities include transmembrane TRAIL undergoing oligomerization at the cell membrane following binding to its receptor on target cells.
The present study examined the possible therapeutic value of an adenovirus encoding TRAIL gene for the treatment of malignant glioma. This virus has previously been shown to kill melanoma, breast carcinoma cell lines (27), prostate and bladder carcinoma cells (31), and lung, colon cancer cell lines (32). In this study, we demonstrated that Ad.hTRAIL induces cell death of malignant glioma. Our data confirm recent studies demonstrating antitumor activity of Ad.hTRAIL, and suggest TRAIL gene transfer as a potential alternative strategy for the efficient delivery of TRAIL.
Figures and Tables
 | Fig. 1Production of adenovirus-encoding human TRAIL. (A) Ad.hTRAIL-infected U87MG cells express TRAIL protein according to MOI and period for incubation. Cell lysates from uninfected or Ad.hTRAIL-infected U87MG cells were prepared 24 hr after infection with different MOI (upper panel) and different incubation period with 300 MOI (lower panel). (B) Ad.hTRAIL-infected U87MG cells secret TRAIL into the media and secreted TRAIL was quantified by ELISA. |
 | Fig. 2Death of glioma cells after Ad.hTRAIL infection results from increased production of TRAIL protein. (A) U87MG cells. (B) U373MG cells. 96 well plates were seeded with 5×104 cells/well and allowed to adhere for at least 12 hr before infection with Ad.hTRAIL or Ad.lacZ at the indicated number of MOI. Cell viability was determined after 24 hr by MTT. Results shown are the mean±SEM from at least three independent experiments. *p<0.05, †p<0.01. |
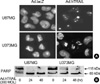 | Fig. 3Ad.hTRAIL-infected glioma cells undergo apoptotic cell death. (A) Nuclear fragmentation was examined by Hoechst nuclear staining in U87MG and U373MG cells treated with Ad.hTRAIL or Ad.lacZ. (B) Cleavage of PARP from 118 to 85 kDa occurs during apoptotic cell death. Cell lysates were prepared of various times after infection with 300 MOI and cellular proteins were determined by Western blot analysis. |
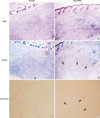 | Fig. 4In vivo assessment of cell death by intratumoral delivery of Ad.hTRAIL. Ten days after tumor implantation, Ad.hTRAIL was administered by intratumoral injection. After 3 days, the tumor regions was excised and analyzed. (A & B) Tumor tissues were stained with H&E (×100) and Ad.lacZ-infected tissue (A) show well preserved and proliferated tumor cells. Ad.hTRAIL-infected tissue (B) showes markedly reduced tumor cells in the center and only small numbers of the cells are present at periphery. (C & D) TUNEL assay demonstrates that Ad.hTRAIL-infected cells (D) show positive stain for apoptotic cells at periphery and that Ad.lacZ-infected cells (C) are not stained for apoptotic cells (×100). (E & F) Immunohistochemistry using anti-TRAIL antibody shows that Ad.hTRAIL expressed TRAIL protein in vivo in Ad.hTRAIL-infected tumor cells (F). Ad.lacZ-infected tumor cells (E) are not detected by anti-TRAIL antibody. |
 | Fig. 5Ad.hTRAIL therapy significantly suppresses the growth of established tumors. Male SCID mice were injected subcutaneously with 1×106 U87MG cells. Animals were then given an injection of Ad.lacZ or Ad.hTRAIL at the site of tumor implantation on day 7. Tumor volume (µL) was monitored every other day for 30 days. Animals treated with Ad.hTRAIL have significantly smaller tumors than Ad.lacZ-treated animals (*p<0.05). |
References
1. Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995. 3:673–682.

2. Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 Ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996. 271:12687–12690.

3. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999. 104:155–162.

4. Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-relatel apoptosis-inducing ligand in vivo. Nat Med. 1999. 5:157–163.
5. Gliniak B, Le T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 1999. 59:6153–6158.
6. Roth W, Isenmann S, Naumann U, Kugler S, Bahr M, Dichgans J, Ashkenazi A, Weller M. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999. 265:479–483.

7. Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000. 97:1754–1759.

8. Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997. 276:111–113.

9. Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997. 277:818–821.

10. Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997. 16:5386–5397.

11. MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997. 272:25417–25420.

12. Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997. 186:1165–1170.

13. Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NFκB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997. 7:813–820.

14. Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997. 7:1003–1006.

15. Pan G, Ni J, Yu G, Wei YF, Dixit VM. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signaling. FEBS Lett. 1998. 424:41–45.
16. Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS, Linet MS. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999. 91:1382–1390.
17. Rieger J, Naumann U, Glaser T, Ashkenazi A, Weller M. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 1998. 427:124–128.

18. Wu M, Das A, Tan Y, Zhu C, Cui T, Wong MC. Induction of apoptosis in glioma cell lines by TRAIL/Apo-2L. J Neurosci Res. 2000. 61:464–470.

19. Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000. 60:847–853.
20. Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999. 162:2639–2647.
21. Kobayashi K, Oka K, Forte T, Ishida B, Teng B, Ishimura-Oka K, Nakamuta M, Chan L. Reversal of hypercholesterolemia in low density lipoprotein receptor knockout mice by adenovirus-mediated gene transfer of the very low density lipoprotein receptor. J Biol Chem. 1996. 271:6852–6860.

22. McGrory WJ, Bautista DS, Graham FL. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988. 163:614–617.

23. Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977. 36:59–74.

24. Teng B, Blumenthal S, Forte T, Navaratnam N, Scott J, Gotto AM Jr, Chan L. Adenovirus-mediated gene transfer of rat apolipoprotein B mRNA-editing protein in mice virtually eliminates apolipoprotein B-100 and normal low density lipoprotein production. J Biol Chem. 1994. 269:29395–29404.

25. Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-κB pathway. Immunity. 1997. 7:821–830.

26. Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity. 1997. 7:831–836.

27. Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998. 161:2833–2840.
28. Weller M, Kleihues P, Dichgans J, Ohgaki H. CD95 ligand: lethal weapon against malignant glioma? Brain Pathol. 1998. 8:285–293.

29. Naumann U, Waltereit R, Schulz JB, Weller M. Adenoviral (full-length) Apo2L/TRAIL gene transfer is an ineffective treatment strategy for malignant glioma. J Neurooncol. 2003. 61:7–15.
30. Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O'connell M, Kelley RF, Ashkenazi A, de Vos AM. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999. 4:563–571.
31. Griffith TS, Broghammer EL. Suppression of tumor growth following intralesional therapy with TRAIL recombinant adenovirus. Mol Ther. 2001. 4:257–266.

32. Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, Curley SA, Stephens LC, Fang B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001. 61:3330–3338.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download