Abstract
Intradermal gene administration was found to induce a more profound immune response than direct intramusclular gene injection. We performed intradermal vaccination of B10.PL mice with DNA encoding for the Vβ8.2 region of the T-cell receptors (TCR). Three weeks later, these mice were immunized with rat myelin basic protein (MBP). Daily mean clinical scores and mortality rate were lower in this group compared with controls. The proliferative responses of lymph node cells to rat MBP were slightly less in the vaccination groups than in the control groups (p<0.05). However, we detected no differences between the two groups with regard to the production of MBP-specific IgG, IgG1, & IgG2a antibodies. The levels of cytokine mRNA expression in the vaccination groups were observed higher than in the control groups without antigen-specific stimulation, but all of cytokine expressions between the vaccination and control groups after antigen-specific stimulation were identical. These results demonstrate that intradermal DNA vaccines encoding for TCR might prove to be useful in the control of autoimmune disease.
T cells, which are able to recognize myelin basic protein (MBP), are thought to play a major role in the pathophysiology of experimental autoimmune encephalomyelitis (EAE). They are activated in the periphery, and then migrate to the central nervous system (CNS), inducing autoimmune inflammation culminating in motor paralysis (1). B10PL mice, when immunized with the acetylated N-terminal MBP peptide (MBP NAc 1-9), were found to generate encephalitogenic CD4+ T cell clones, which then migrated to the CNS, inducing EAE. Approximately 85% of encephalitogenic clones express the T cell receptor (TCR) Vβ8.2 (2).
Therefore, an ideal therapy for EAE could involve selective inactivation, elimination, or functional deviation of the TCR Vβ8.2. Monoclonal antibodies targeted to pathogenic V gene products, as well as T-cell vaccination using peptides from the complementarity-determining regions of the pathogenic TCR V region, can induce resistance to the development of EAE (3). Unfortunately, peptide-based vaccines are poor immunogens (4). Genetic vaccinations with naked DNA have been reported to result in long-lasting cellular and humoral immune responses (5), and also, promote a shift in the pattern of cytokines produced by the pathogenic T cells (6). Previous studies have indicated that intramuscular injection of Vβ8.2 DNA in PL/J mice did not exhibit suppression of EAE development when they were immunized with whole MBP (7). The possibility exists that muscle was not considered to be a viable site for antigen presentation, as it contains few, if any, dendritic cells, macrophages, and lymphocytes (8). Furthermore, it has been well established that skin-associated lymphoid tissues harbor specialized cells which enhance immune responses, and that intradermal gene administration induces more profound immune responses than does intramuscular gene delivery (5). Therefore, the in vivo transfection of epidermal or dermal cells with DNA would be expected to constitute an efficient route for gene immunization. This study was undertaken to determine the suppressive effects of intradermal gene vaccination with plasmid DNA encoding for Vβ8.2 on the clinical outcome of EAE after immunization with whole rat MBP.
The 40 female B10.PL mice used in this study were obtained from The Jackson Laboratories (Bar Harbor, ME, U.S.A.), and bred at the Memphis Veterans Administration Medical Center Animal Research Facility. The animals were between 6 and 8 weeks old at the time of disease induction.
MBP was prepared from frozen rat brains (Pel-Freez Biological, Rogers, AR) by the method developed by Smith (9). In brief, the dissected rat brains were homogenized in 0.32 mol/L sucrose, then layered onto 0.85 mol/L sucrose. After centrifugation (25,00 rpm for 30 min), the interface was carefully removed, washed three times with deionized water, and lyophilized overnight. The protein was then resuspended in 90% acetone and stirred for 30 min at room temperature. The precipitate was dried and redissolved in 10 mL 0.3 N hydrochloric acid. The supernatant was carefully removed, and dialyzed against 10 mM/L acetic acid. The acetic acid was removed by lyophilization, and the MBP concentration levels were deduced by 12% sodium dodecyl sulfate gel electrophoresis. The SDS gel was stained with Coomassie Brilliant Blue R250 solution, and destained in acetic acid/methanol (10% and 45% v/v, respectively). The total concentration of the three major bands in the MBP protein (~16, ~19, and ~21 kDa) were estimated based on the concentrations of standard protein marker (GibcoBRL, Life Technologies, Gaithersburg, MD, U.S.A.).
cDNA from the 147 clone was used in this study (2). This cDNA was employed in PCR using Taq polymerase (Stratagene, LaJolla, CA, U.S.A.) with primers specific for Vβ8.2 (5'-CCG GAA TTC GCC GCC ACC ATG CAC ATG GAG GCT GCA GTC ACC CAA-3'and 5'-TGC TCT AGA TTA GCT GGC ACA GAA GTA CAC TGA-3'). These primers encompassed the entire V region (about 320 bp), and included the EcoRI and XbaI sites used in cloning. The amplified PCR products were subsequently cloned into the pcDNA3.1 eukaryotic expression vector (Invitrogen, San Diego, CA, U.S.A.), and sequenced, in order to verify the insertion of the right gene into the appropriate open reading frame.
Plasmid construct was prepared using Maxi Prep (Quiagen, Chatsworth, CA, U.S.A.). There were two groups and each group consists of twenty mice. B10.PL mice were injected intradermally with 1-mL syringes and 28-gauge needles. Intradermal inoculation was carried out 1-2 cm distal from the tail base, with 300 µg of pcDNA3.1 blank vector suspended in 100 µL PBS (control group), or an equal amount of the mixed naked DNA encoding for the TCRVβ8.2 (vaccination group). Injections took place three times, at weekly intervals.
EAE was induced by a modification of the standard protocol (10). Twenty B10.PL mice of each group received subcutaneous injections of 200 µg of rat MBP and killed Mycobacterium tuberculosis (H37RA, Difco, Detroit, MI, U.S.A.), at a concentration of 3 mg/mL, in a 0.1-mL emulsion consisting of equal volumes of 10 mM/L acetic acid and complete Freund's adjuvant (CFA). 300 ng of Bordetella pertussis toxin (List Pharmaceuticals, Campbell, CA, U.S.A.) was administered intraperitoneally 24 and 72 hr after MBP immunizations. The mice were then observed daily for signs of EAE. The scale used to grade the clinical status of the diseased mice was as follows: 0, no clinical disease; 1, limp tail or hind limb weakness; 2, limp tail and hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; 5, moribund state. The mean clinical score in the study was defined as the mean clinical score of all the animals. Mortality was expressed as the percentage of animals that died as the result of severe clinical EAE. The mean day of onset indicates the mean day of the inception of signs of acute clinical disease in the mice exhibiting clinical signs. The mean peak of disease severity was evaluated as the mean of the highest clinical score achieved by the mice exhibiting clinical signs.
Blood was collected from both groups of mice three times, at weeks 0 (first immunization), 2, and 3. The rat MBP-specific IgG, IgG1, and IgG2a antibody responses were assessed by ELISA, as follows. One hundred microliters of rat MBP (5 µg/mL in 0.1 M carbonate buffer, pH 9.6) were dispensed into each well of a polystyrene microtiter plate (Costar, Cambridge, MA, U.S.A.), then incubated overnight at 4℃. The antigen-coated plates were washed three times in 0.05% PBS-Tween 20 buffer (washing buffer), and incubated with mice sera at 4℃ overnight. The plates were then washed five times with washing buffer, and incubated with peroxidase-conjugated anti-mouse IgG antibody, goat anti-mouse IgG1, and IgG2a (Sigma, St. Louis, MO, U.S.A.) overnight at 4℃. The plates were then washed five times, prior to the addition of citric acid-phosphate buffer (pH 5.0) containing 0.15 mg/mL of O-phenylenediamine (Sigma). The color was developed at room temperature, and the reaction was discontinued via the addition of 2.5 M sulfuric acid. The color was the evaluated at a wavelength of 492 nm (Bio-Rad, Richmond, CA, U.S.A.).
The proliferation assay was performed as described previously (11). The lymph nodes were removed 10 days after immunization with rat MBP, and single-cell suspensions were prepared. The cells (2×105 cells per well) were then cultured with serial dilutions of rat MBP (range, 0.01-10 µg/mL). Cultures were constructed in 200 µL of RPMI1640, supplemented with 10% fetal calf serum (Hyclone Laboratories, Logan, UT, U.S.A.), 1 mM/L sodium pyruvate, 100 µg/mL penicillin, 100 g/mL streptomycin, 2 mM/L glutamine, 5×10-5 M/L 2-mercaptoethanol, 20 mM/L HEPES (pH 7.4), and 50× nonessential amino acids. After 4 days of culturing, 1 µCi of [3H] thymidine (Du Pont, Wilmington, DE, U.S.A.) was added to each well. 18 hr later, the cells were harvested and measured via the liquid scintillation counting method. Values were expressed in counts per minute, as follows: Counts per minute with antigen - Counts per minute without antigen. Each sample was assessed in triplicate in this manner. RPMI medium 1640, sodium pyruvate, penicillin, streptomycin, glutamine, HEPES, and 50× nonessential amino acids were purchased from Irvine Scientific (Santa Ana, CA, U.S.A.), and 2-mercaptoethanol was purchased from the Sigma Chemical Co.
Lymph node cells (1×107 cells per well) were cultured in the presence of rat MBP (100 µg/mL) in vitro for 18 hr. The cells were washed with PBS buffer, and total RNA was extracted with TRIzol Reagent (Biotecx, Houston, TX, U.S.A.). By using murine leukemia virus reverse transcriptase and random hexanucleotide primer, according to the instructions provided in the Perkin-Elmer Gene Amp RNA PCR kit (Perkin Elmer, Branchberg, NJ, U.S.A.), we generated first-strand cDNA from 1 µg of total RNA, and subjected it to RT-PCR analysis. In order to determine the relative abundance of the mRNA expression of each cytokine, the amount of each cDNA for PCR was optimized according to the intensity of the amplified DNA β-actin products generated from each RNA. In the PCR reaction mixture, we added either β-actin as a control primer, IL-2, IFN-γ (Clonetech, PaloAlto, CA, U.S.A.), IL-4, or IL-5, at a final concentration of 0.2 µM. The sequence of these primers is summarized in Table 1. The PCR conditions were as follows: 200 µM of dNTP, 10 µCi [32P] dCTP, 50 µM Tris. HCl (pH 9.0), 50 µM NaCl, 2 µM MgCl2, 0.5 mM DTT, and two units of Taq polymerase (Perkin Elmer) at a final volume of 20 µL. A negative control reaction was conducted with each sample, in order to verify that no PCR bands appeared in the absence of the template. The optimal amplification conditions were as follows: denaturation for 45 sec at 94℃, annealing for 45 sec at 67℃, and elongation for 1 min at 72℃. Our PCR protocol called for 30 cycles. The amplified DNAs of β-actin, IFN-γ, IL-2, IL-4, and IL-5, were 540, 365, 413, 354, 349, and 455 bp in length, respectively. The gel was dried on Whatman 3M paper, then exposed to Kodak XAR film. In each run of electrophoresis, intra- and inter-gel staining homogeneity was verified via assessments of the staining intensities of the molecular weight markers at both ends of the gels. In general, the amplification kinetics for each PCR run were monitored via assessments of aliquots of the gel products. Amounts of PCR products were compared during the cycle in which the amplification did not achieve saturation.
Significance of the differences in results was determined by Student's t-test for immunoglobulin production and proliferation assays. In order to determine the severity of disease, we utilized the Mann-Whitney test. A p value of <0.05 was considered to be statistically significant.
The control group developed a more severe course of clinical EAE than did the vaccination group. This was reflected by higher daily mean clinical score (Fig. 1) and mortality rate (50% vs. 0%) in the control group, after immunization with whole MBP. However, no differences were observed between groups with regard to the mean day of onset (21.67±5.35 days vs. 18.67±3.88 days), or the mean peak severity of disease (3.83±1.47 days vs. 2.51±0.54 days). These data suggested that intradermal vaccination with Vβ8.2 might exert a partially preventive effect on the induction of EAE disease, after immunization with whole MBP.
IgG1 antibody depends on the interleukin (IL)-4 which is secreted by the Th2 cells (12). Intradermal vaccination with Vβ8.2 DNA (6.75±1.36 µg/mL, 7.03±1.51 µg/mL) resulted in no different response with regard to MBP-specific IgG1 antibody, as compared with the control group (6.34±1.08 µg/mL, 6.34±0.86 µg/mL) at 2 and 3 weeks post-immunization with whole MBP (p>0.05). IgG2a antibody formation is dependent on interferon-γ(IFN-γ) as an IgM-to-IgG2a switching factor, and is believed to be a typical Th1 response (12). In regard to the response of MBP-specific IgG2a antibodies, the vaccination group (14.65±1.85 µg/mL, 14.64±1.98 µg/mL) did not significantly increase antibody production, in comparison with the control group (12.66±1.06 µg/mL, 12.80±1.00 µg/mL) (p>0.05). Also there were no difference between the vaccination (1.41±0.16 mg/mL, 1.27±0.22 mg/mL) and control groups (1.40±0.06 mg/mL, 1.39±0.06 mg/mL) with regard to the response of MBP-specific total IgG antibodies (p>0.05). Thus, intradermal genetic vaccination did not result in any differences from the control group in terms of the production of antibodies after immunization (Fig. 2).
Even though the levels of mRNA expression of IFN-γ, IL-2, IL-4, & IL-5 in the vaccination group were observed higher than in the control group without antigen-specific stimulation, we detected no differences all of the cytokine expressions between the two groups after antigen-specific stimulation (Fig. 3). These data suggest that intradermal DNA vaccination with Vβ8.2 DNA might not exert any significant effects on the shift from the Th1-type cytokine to the Th2-type cytokine.
On day 10 after immunization with the rat MBP in CFA, the vaccination group's proliferative responses to MBP of lymph node cells was found to be significantly less pronounced than in the control group at antigen concentration 0.01 µg/mL, 0.1 µg/mL, 1 µg/mL, 100 µg/mL (5082.08±933.21, 6759.33±1857.77, 10019.83±1494.50, 28228.83±1699.82 vs. 10155.50±2096.45, 11504.49±718.14, 14861.50±1174.39, 32003.00±1734.84 dpm, p<0.05), except 10 µg/mL (14174.08±3337.39 vs. 18936.50±2201.55 dpm, p>0.05) (Fig. 4). Intradermal vaccination with DNA encoding for TCR Vβ8.2 might induce the suppression of lymph node cell proliferation, in response to MBP.
The potential advantages associated with DNA vaccination include the prolonged, endogenous expression of antigens (5), long-term immunity with efficient generation of both CD8+ cytotoxic T cells and CD4+ T helper cells (6, 7), and the possibility of modulating Th1 or Th2 responses via the alteration of vaccination protocols (13). It has also been established that the in vivo transfection of the epidermis or dermal cells by DNA would be expected to provide an efficient route for gene immunization, due to immune response. The present study sought to evaluate the effects of intradermal vaccination with DNA encoding for TCR Vβ8.2, on the induction of EAE in B10.PL mice, after the mice had been immunized with whole MBP. This study appears to have been extremely effective with regard to the induction of EAE after immunization, as reflected by the mean daily scores and mortality rate, even if the T-cell recognition of MBP p35-47 does not involve the Vβ8.2 gene product (14). The results of our present study confirm the results of an earlier study, which reported that intradermal gene administration induced a more profound immune response than did intramuscular gene delivery (5).
Although immunization with the TCR peptide (3) and vaccination with DNA (7, 15) also resulted in some protective effects on EAE development, little information is currently available regarding the mechanisms of action underlying the protection induced by DNA vaccination. A previous study reported that DNA vaccination resulted in a Th1 to Th2 cytokine shift, and then the Th2 cytokines suppressed EAE development (7). However, another previous study demonstrated that there were no signs of a Th2 cytokine bias, even if DNA vaccination did, indeed, suppress the development of EAE (16). Furthermore, myelin/oligodendrocyte glycoprotein-induced EAE was aggravated after the induction of Th2 immunity (17). In addition, IL-4-deficient mice, which exhibit defective Th2 responses, could be tolerant to the induction of EAE (18). The results of a RT-PCR analysis on the total RNA samples extracted from the lymph node cells also demonstrated that vaccination did not induce a Th1-to-Th2 cytokine shift. In the case of MBP-induced immune response, we determined there to be no difference between the vaccination and control groups with regard to the response of IgG1 & IgG2a antibodies in the present study. This study indicated that intradermal vaccination with DNA encoding for TCR Vβ8.2, in the case of EAE, did not induce a shift in cytokine response from Th1 to Th2. Our findings were consistent with other recent studies, which reported that the shift from Th1 to Th2 cytokine responses is not directly involved in protective effects against EAE.
In order to determine whether the observed protective effects of gene vaccination were attributable to the deletion of T cells or to the induction of unresponsiveness, we examined the proliferation of lymph node cells in proportion to the MBP concentrations. In our study, we determined that T cell proliferative responses against MBP in the vaccination group were partially suppressed, compared to those in the control group, but were higher in proportion to the MBP concentration. T cell deletion has been observed primarily after the administration of high doses of antigens or peptides (19), and the presentation of antigens by nonprofessional antigen-presenting cells (APCs) which lack co-stimulatory capacity results in anergy, rather than in priming (20). However, professional APCs, Langerhans cells, or macrophages, all of which are abundant in the dermis, may also function as APCs after intradermal DNA vaccinations (5). It is also possible that the intradermal gene vaccination in our study might not have induced either T cell deletion or anergy.
Recently, it has been suggested that immunization with a tolerogenic form of the antigen induces the generation of regulatory CD4+CD25+ T cells, which, when activated either by the antigen or by anti-idiotype-bearing transducer suppressor cells, then served to mediate downmodulating T-helper effect functions on other antigens present in conjunction with the immunogen (21). However, the specificity or mechanisms associated with regulatory CD4+ T cell-mediated suppression are not currently well understood. It has been reported, from earlier studies, that DNA vaccines are efficient inducers of regulatory CD8+ T cell-mediated immunity, and that CD8+ and MHC I-restricted effector T cells ultimately inhibit the function of encephalitogenic Vβ8.2+ T cells (22, 23). Also, it is clear that the priming of the regulatory CD4 T cells must occur in a Th1 milieu for the effective regulation of anti-MBP responses, as well as protection from EAE. Furthermore, if the regulatory CD4+ T cells are compelled to deviate in the direction of Th2 direction, this would result in the exacerbation of the disease, due principally to the absence of the natural regulation provided by MBP-reactive T cells (24). Recent reports suggest that protection from EAE after DNA vaccination is predicated on immunostimulatory, unmethylated CpG DNA motifs, and also that the underlying protective mechanism of DNA vaccination involves the immunomodulatory effects which are exerted by the induced IFN-β, with no evidence for a shift in immune response toward the production of type 2 cytokines (25). Also, IFN-β exerts anti-proliferative effects on peripheral blood monocytes. Our result demonstrated that the levels of mRNA expression of IFN-γ, IL-2, IL-4, & IL-5 in the vaccination group were observed higher than in the control group without antigen-specific stimulation, but there were no differences in all of the cytokine expressions between the two groups after antigen-specific stimulation. This is consistent with the reports showing that DNA vaccination activated T cells, B cells and dendritic cells to produce a wide range of cytokines, but, DNA vaccination induced the immunomodulation effect on the EAE after antigen-specific stimulation (25). Therfore, we have determined that intradermal DNA vaccination does not result in a shift from Th1 to Th2 cytokine responses, and also that T cell proliferative responses against MBP after DNA vaccination were suppressed, as compared to what we observed in the control group. This is consistent with earlier observations (25). Although the observed protective autoimmune mechanisms underlying DNA vaccination in our study have yet to be clearly established, we suggest that the mechanisms underlying intradermal vaccination with Vβ8.2 DNA might be associated with the immunomodulation exerted by the induced IFN-β.
In summary, genetic immunization is associated with several advantages as compared with conventional vaccines, in that genetic immunization makes it possible to express antigens in forms which promote natural processing and presentation to lymphocytes. Furthermore, DNA vaccines are safe, stable, and induce long-lasting responses in host. Our present study demonstrates that intradermal vaccination with DNA encoding for the Vβ8.2 gene of the T-cell receptor might exert inhibitory effects on the induction of EAE in mice. This study suggests that intradermal vaccination with DNA encoding for TCR might constitute a useful tool in the control of autoimmune disease.
Figures and Tables
Fig. 1
Preventive effects of DNA vaccination with TCR Vβ8.2 in mice after active immunization with rat MBP on the induction of EAE disease. The vaccination group (n=20) was intradermally vaccinated with DNA encoding for the Vβ8.2 of the T-cell receptor, three times at weekly intervals. The control group (n=20) was injected with pcDNA 3.1 blank vector. After 3 weeks, all mice were immunized with rat MBP. The clinical symptoms of EAE were monitored and scored daily, as described in the Methods section. The control group developed higher daily mean clinical score than the vaccination group between days 20-25 (1.50±0.92, 1.63±0.74, 2.25±1.28, 3.0±1.07, 3.38±1.19, 3.5±1.41 vs. 0.13±0.35, 0.38±0.52, 0.50±0.76, 1.00±1.07, 1.25±1.06, 1.13±1.36, p<0.05). There was a dramatic reduction in mortality in the vaccination group compared with controls; eight of sixteen mice died in the control group, compared with none in the vaccination gorup (p<0.05).

Fig. 2
The IgG1 (A), IgG2a (B), and IgG (C) antibody responses of the B10.PL mice immunized with rat MBP with DNA coding for Vβ8.2 or pcDNA only. Intradermal vaccination with Vβ8.2 DNA resulted in no different response with regard to MBP-specific IgG1 antibody, as compared with the control group, at 2 and 3 weeks post-immunization with whole MBP (p>0.05). Also, with regard to the response of MBP-specific IgG2a and IgG antibodies, the vaccination group did not significantly increase antibody production, in comparison with the control group (p>0.05). Sera of eigth mice in each group were collected three times on weeks 0, 2, and 3, after immunization. Data are expressed as means±S.D.

Fig. 3
Cytokine gene expression. Four mice from each group were sacrificed 10 days-after MBP immunization. Lymph node cells (1×107 cells per well) were cultured either without antigen, or with rat MBP (100 µg/mL) in vitro, for 18 hr. The cells were washed with PBS buffer and the total RNA was extracted using TRIzol reagent. Using murine leukemia virus reverse transcriptase and random hexanucleotide primer, according to the instructions of the Perkin Elmer Gene Amp RNA PCR kit, first-strand cDNA was generated from 1 µg of total RNA and subjected to RT-PCR analysis. RT-PCR reactions were then conducted using cDNA with different primers, specific for β-actin, IL-2, γ-interferon, IL-4, and IL-5. (1) Lymph node cells from the control mice were cultured without antigen, (2) or in the presence of rat MBP (100 µg/mL). (3) Lymph node cells from the vaccinated mice were cultured without antigen, (4) or in the presence of rat MBP (100 µg/mL). The levels of mRNA expression of IFN-γ, IL-2, IL-4, & IL-5 in the vaccination groups were observed higher than in the control groups without antigen-specific stimulation, but there were no differences all of the cytokine expressions between the two groups after antigen-specific stimulation.
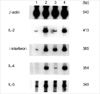
Fig. 4
Lymph node cell proliferation 10 days after immunization, the lymph nodes of four mice of each group were removed aseptically, and single-cell suspensions were prepared. The cells (2×105 cells per well) were then cultured with serial dilutions of rat MBP (range, 0.01-100 µg/mL). After 4 days of culturing, 1 µCi of [3H] thymidine was added to each well. 18 hr later, the cells were harvested, and measured via liquid scintillation counting. The vaccination group's proliferative responses to MBP of lymph node cells was found to be significantly less pronounced than in the control group (p<0.05 at antigen concentrations 0.01-100 µg/mL, except 10 µg/mL). The values were expressed in counts per minute, as follows: Counts per minute with antigen - Counts per minute without antigen. *p<0.05 vaccination group compared with control group.

References
1. Mor F, Kantorowitz M, Cohen IR. The dominant and the cryptic T cell repertoire to myelin basic protein in the Lewis rat. J Neurosci Res. 1996. 45:670–679.

2. Cheng KC, Chiang HJ, Wang K, Krug MS, Yoo TJ, Hood L. TCRV and TCRJ gene usage in MBP responding T cells from (B10.PL×PL/J) F1 mice is biased towards that of B10.PL mice. J Neuroimmunol. 1997. 80:13–22.
3. Matsumoto Y, Tsuchida M, Hanawa J, Abo T. T cell receptor peptide therapy for autoimmune encephalomyelitis: stronger immunization is necessary for effective vaccination. Cell Immunol. 1994. 153:468–478.

4. Bot A, Bot S, Antohi S, Karjalainen K, Bona C. Kinetics of generation and persistence on membrane class II molecules of a viral peptide expressed on foreign and self proteins. J Immunol. 1996. 157:3436–3442.
5. Raz E, Carson DA, Parker SE, Parr TB, Abai AM, Aichinger G, Gromkowski SH, Singh M, Lew D, Yankauckas MA, Baird SM, Rhodes GH. Intradermal gene immunization: The possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994. 91:9519–9523.

6. Kwon SS, Kim N, Yoo TJ. The effect of vaccination with DNA encoding murine T-cell epitopes on the Der p 1 and 2 induced immunoglobulin E synthesis. Allergy. 2001. 56:741–748.

7. Waisman A, Ruiz PJ, Hirschberg DL, Gelman A, Oksenberg JR, Brocke S, Mor F, Cohen IR, Steinman L. Suppressive vaccination with DNA encoding a variable region gene of the T-cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nature Med. 1996. 2:857–859.

8. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991. 9:271–296.

10. Watanabe T, Cheng KC, Krug MS, Yoo TJ. Brain stem auditory-evoked potentials of mice with experimental allergic encephalomyelitis. Ann Otol Rhinol Laryngol. 1996. 105:905–915.

11. Chen KC, Lee KM, Krug MS, Watanabe T, Suzuki M, Choe IS, Yoo TJ. Houst dust mite-induced sensitivity in mice. J Allergy Clin Immunol. 1998. 101:51–59.
12. Gorelik L, Flavell RA. Abrogation of TGF-beta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000. 12:171–181.
13. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990. 247:1465–1468.

14. Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995. 32:121–182.

15. Lobell A, Weissert R, Storch MK, Svanholm C, de Graaf KL, Lassmann H, Andersson R, Olsson T, Wigzell H. Vaccination with DNA encoding an immunodominant myelin basic protein peptide targeted to Fc receptor of immunoglobulin G suppresses experimental autoimmune encephalomyelitis. J Exp Med. 1998. 187:1543–1548.
16. Genain CP, Abel K, Belmar N, Villinger F, Rosenberg DP, Linington C, Raine CS, Hauser SL. Late complications of immune deviation therapy in a nonhuman primate. Science. 1996. 274:2054–2057.

17. Marusic S, Tonegawa S. Tolerance induction and autoimmune encephalomyelitis amelioration after administration of myelin basic protein-derived peptide. J Exp Med. 1997. 186:507–515.

18. Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994. 1:365–371.

19. Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998. 19:89–97.

20. Kumar V, Sercarz E. Self-determinant selection and selective regulation. Chem Immunol. 1995. 60:1–19.

21. Furtado GC, Olivares-Villagomez D, Curotto de Lafaille MA, Wensky AK, Latkowski J, Lafaille JJ. Regulatory T cells in spontaneous autoimmune encephalomyelitis. Immunol Rev. 2001. 182:122–134.

22. Mathisen PM, Tuohy VK. Gene therapy in experimental autoimmune encephalomyelitis. J Clin Immunol. 2000. 20:327–333.
23. Jiang H., Curran S, Ruiz-Vazquez E, Liang B, Winchester R, Chess L. Regulatory CD8+ T cells fine-tune the myelin basic protein-reactive T cell receptor Vβrepertoire during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2003. 100:8378–8383.
24. Braciak TA, Pedersen B, Chin J, Hsiao C, Ward ES, Maricic I, Jahng A, Graham FL, Gauldie J, Sercarz EE, Kumar V. Protection against experimental autoimmune encephalomyelitis generated by a recombinant adenovirus vector expressing the Vβ 8.2 TCR is disrupted by coadministration with vectors expressing either IL-4 or IL-10. J Immunol. 2003. 170:765–774.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download