Abstract
Major ABO incompatibility may be potentially associated with immediate or delayed hemolysis and delayed onset of erythropoiesis in patients receiving allogeneic hematopoietic stem cell transplantation (HSCT). To determine if hemolysis can be prevented by the inhibition of graft erythropoiesis, we performed hypertransfusion and assessed red cell transfusion requirement and independence. Between October 1995 and December 2001, 28 consecutive patients receiving major ABO incompatible HSCT at Samsung Medical Center were hypertransfused to maintain their hemoglobin levels at 15 g/dL or more. We retrospectively compared the outcomes of these patients with those of 47 patients at Asan Medical Center whose target hemoglobin levels were 10 g/dL. Reticulocyte engraftment was significantly delayed in hypertransfused group (51 days vs. 23 days; p=.001). There was no significant difference in the total amount of red cells transfused within 90 days post-HSCT (25 units vs. 26 units; p=.631). No significant difference in the time to red cell transfusion independence was observed between the two groups (63 days vs. 56 days; p=.165). In conclusion, we failed to improve red cell transfusion requirement and independence in major ABO incompatible HSCT with hypertransfusion.
Allogeneic hematopoietic stem cell transplantation from HLA-matched or unrelated donors has a high curative potential in various hematologic diseases. ABO antigens are inherited independently from the HLA antigen complex (1). Major ABO incompatibility means the recipient has isoagglutinin capable of reacting with ABO antigens on the surface of donor red cells. It has been known that approximately 15 to 20 percent of stem cell recipients have a major ABO incompatibility with their donors, yet ABO incompatible stem cell transplantation has not previously been proven to be associated with decreased survival (2, 3).
However, the graft stem cell product contains donor type red cells approximately the same volume as a unit of whole blood. The red cell mass transfused is sufficient to precipitate a severe hemolytic reaction at the time of their infusion. Hemolysis at graft infusion may be avoided by red cell or plasma depletion of the graft (4) or by in vivo depletion of recipient isoagglutinin (5, 6).
In addition to the risk of acute hemolysis at the time of infusion of the donor stem cells, major ABO incompatible stem cell transplantation carries the potential risks of delayed onset of hemolysis and delayed onset of erythropoiesis (7, 8), resulting in prolonged transfusion dependency and increased red cell transfusion requirement. The engrafted stem cells continue to produce the donor type red cells and these incompatible red cells substantiate the risk of delayed hemolytic reactions. Thus we hypothesized that the risk of delayed hemolysis in major ABO incompatible stem cell transplantation could be reduced with the suppression of graft erythropoiesis while recipient isoagglutinin remained at a significant titer.
To further suppress graft erythropoiesis, we performed hypertransfusion. Hypertransfusion is a technique used for patients with thalassemia syndrome, based on the reduction of erythropoiesis and evidenced by the decreased number of reticulocytes (9). This study examined a variety of factors to confirm whether we could reduce the risk of hemolysis with hypertransfusion.
Between October 1995 and December 2001, 28 consecutive adult patients receiving major ABO incompatible (including bi-directional and pure major ABO mismatches) allogeneic hematopoietic stem cell transplantation at Samsung Medical Center were hypertransfused to maintain their hemoglobin levels at 15 g/dL or more. Hypertransfusion continued until their isoagglutinin titers became 1:8 or less. We retrospectively compared the outcomes of these hypertransfused patients with those of 47 patients at Asan Medical Center whose target hemoglobin levels were 10 g/dL.
All patients received white cell-depleted and irradiated group O red cells. ABO compatible platelets were given preferentially if available. Graft stem cells were infused after red cell depletion using a cell separator (COBE-2991 cell processor, COBE BCT, Lakewood, CO) in both centers. All patients were nursed in the single isolation room with laminar air filter.
Complete blood counts with differential and reticulocyte counts were measured daily starting 7 days before transplantation until sustained hematopoietic engraftment was achieved, then at least weekly until day 100, and from then biweekly up to 1 yr. Bone marrow aspirates and biopsies were performed on days 35, 100, 180 and 365 after transplantation. The titers of anti-A and anti-B isoagglutinins were evaluated before the conditioning regimen was begun, then weekly until stable red cell engraftment was achieved.
Hemolysis could be manifested by a sudden drop in hemoglobin of 1.0 g/dL or more without any evidence of bleeding, increases in bilirubin and lactic dehydrogenase, and decreases in serum haptoglobin concentrations. However, it was difficult to evaluate precisely the incidence of hemolysis using above findings because a variety of complications related to stem cell transplantation such as bleeding, infections, veno-occlusive disease, drug effects, or graft versus host disease mimicked the manifestations of hemolysis. Therefore, we indirectly compared the results of our study group with those of the control group in terms of the red cell transfusion requirements and the duration of red cell transfusion dependence. The duration of red cell transfusion dependence was defined as the number of days from the transplantation to the last red cell transfusion that was followed by 30 days without transfusion.
We also evaluated the time to trilineage engraftments and post transplant day 100 survival. The time of red cell engraftment was defined as the first day on which their reticulocyte count of 1 percent or more was achieved for at least 2 consecutive days. We defined the time of granulocyte and platelet engraftments as the first day on which absolute granulocyte count of 500/µL or more was achieved for at least 3 consecutive days and the first day on which platelet count of 20,000/µL or more was achieved for at least 7 consecutive days without platelet transfusion, respectively.
Pure red cell aplasia was diagnosed when red cell engraftment was not achieved and day 365 bone marrow biopsy showed isolated depletion of erythroid precursors, following the exclusion of red cell alloantibodies, viral or bacterial infection, or relapse.
From the preliminary data of our hospital, recipients with major ABO incompatibility were transfused a median of 16.5 units of red cells by day 100 post transplant, whereas recipients of ABO compatible stem cell transplantation received 10.4 units of red cells. These data showed that the amount of transfused red cells conformed to lognormal distribution. Therefore, we were hoping to decrease the red cell transfusion requirement in the recipients of ABO incompatible hematopoietic stem cell transplantation from 16.5 units down to 10.4 units with hypertransfusion. Assuming the significance level of 0.05 and the power of 0.8, we needed 27 patients.
The association between categorical variables were evaluated using the chi-square test or, if appropriate, the two-sided Fisher's exact test. Continuous variables were expressed as median and range, and compared using Mann-Whitney test, if indicated. A p value of less than 0.05 was considered significant. All analyses were done using SPSS 10.0 software.
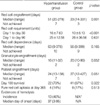
Twenty-eight patients were assigned to the hypertransfusion group, and 47 patients to the control group. Detailed clinical characteristics of these patients are summarized in Table 1. The pre-transplant characteristics of the two groups were essentially similar with respect to age, sex, primary disease, type of donors, and the duration of follow-up. Conditioning and graft versus host disease prophylaxis regimens were allowed to be different between the two groups, according to the physician's preference.
As we had expected, red cell engraftment was significantly delayed with hypertransfusion (51 days in the hypertransfusion group versus 23 days in the control group; p=0.001). During the first 30 days post transplant, significantly more red cells were transfused in the hypertransfusion group (16±7.63 units versus 10±6.13 units; p<0.001). However, there was no significant difference in the total amount of red cells transfused within the first 90 days (25±12.58 units versus 26±36.56 units; p=0.631) (Table 2).
The median duration of the red cell transfusion dependence was 63 days in the hypertransfusion group and 56 days in the control group. No significant difference was observed (p=0.165).
Granulocyte (16 days versus 20 days; p=0.052) and platelet (24 days versus 27 days; p=0.051) engraftments inclined to be faster in the hypertransfusion group, but the p values failed to show any significances.
Comparable day 100 post transplant survival was noted (79 percent versus 87 percent; p=0.322). We evaluated the incidence of pure red cell aplasia at 365 days post transplant and failed to observe any significant difference (14 percent versus 17 percent; p=0.513).
Evidences of hemolysis were evaluated only in the hypertransfusion group, because we found it difficult to evaluate the manifestations of hemolysis in the control group retrospectively. Hemolysis occurred in 46 percent of the patients receiving hypertransfusion.
Among patients receiving major ABO incompatible allogeneic hematopoietic stem cell transplantation, red cell engraftment was delayed with hypertransfusion. However, more red cell transfusion was not required and the duration of red cell transfusion dependence was not prolonged with hypertransfusion.
In a retrospective analysis of 292 allogeneic stem cell transplantation recipients, Benjamin and his colleagues reported that ABO incompatibility resulted in a significantly greater risk of death within 100 days of transplant and delayed onset of red cell engraftment (7). Recipients with major ABO incompatibility received more red cell transfusion during their post-transplant period. Worel et al. also reported of delayed red cell engraftment and significantly decreased day 100 survival in the recipients of major ABO incompatible stem cell transplantation (8). However, still there are controversies whether major ABO incompatibility adversely affects clinical outcomes of transplant recipients (10-12). Major ABO incompatible allogeneic hematopoietic stem cell transplantation has potential immunohematologic complications such as a delay in the onset of erythropoiesis, acute hemolysis at the time of stem cell infusion, or delayed onset of hemolysis associated with persistence of anti-A and/or anti-B after transplantation. Delayed onset of hemolytic reaction stems from the infused red cells of donor type or red cells produced by the engrafted donor stem cells. With the depletion of red cells in graft stem cell product, we could avoid or reduce hemolysis at the time of stem cell infusion. However, it is still evident that the risk of delayed onset of hemolysis is caused by red cells produced by engrafted donor stem cells.
Delayed onset of hemolysis in patients receiving transplantation with red cell depleted stem cell products is rather unexpected since the only source of significant volumes of incompatible red cells would be erythropoiesis by the newly engrafted stem cells unless red cell transfusions of donor type are used in the post-transplant period (13). Nevertheless, Sniecinski et al. reported that 5 out of 58 evaluable patients developed overt evidence of delayed hemolysis (1). We also observed that no fewer than 46 percent of the patients developed evidence of delayed onset of hemolysis in this study.
We started this trial in order to prove the hypothesis that hemolytic complications of major ABO incompatible stem cell transplantation would be reduced with hypertransfusion. It is of note that there was no increase in the total amount of transfused red cells with target hemoglobin level of 15 g/dL. We observed that the red cell transfusion dependence was not prolonged with hypertransfusion. We are not sure whether this is the case, whether it was clinically significant, and whether this might have been due to a reduction in later hemolytic complications.
We do admit this trial has some weaknesses. We failed to compare the overt evidence of hemolysis between the 2 groups. In addition, it is very difficult in allogeneic transplant patients to define a hemolytic event as one manifested by hemoglobin falling ≥1 g/dL in the absence of bleeding with an increase in bilirubin and/or LDH and decrease in serum haptoglobin. All these variables may change for a number of reasons unconnected with hemolysis. Evaluation of the patients who were assigned to the control group was performed retrospectively. There were differences in the type of conditioning regimen and the type of graft versus host disease prophylaxis, even if red cell transfusion requirements and the occurrence of pure red cell aplasia were functions of the type of conditioning regimen and of graft versus host disease prophylaxis. In conclusion, we failed to improve red cell transfusion requirement and independence in major ABO incompatible hematopoietic stem cell transplantation with hypertransfusion.
References
1. Sniecinski IJ, Oien L, Petz LD, Blume KG. Immunohematologic consequences of major ABO-mismatched bone marrow transplantation. Transplantation. 1988. 45:530–534.

2. Lasky LC, Warkentin PI, Kersey JH, Ramsay NK, McGlave PB, McCullough J. Hemotherapy in patients undergoing blood group incompatible bone marrow transplantation. Transfusion. 1983. 23:277–285.

3. Mehta J, Powles R, Horton C, Milan S, Singhal S, Treleaven J. Relationship between donor-recipient blood group incompatibility and serum bilirubin after allogeneic bone marrow transplantation from HLA-identical siblings. Bone Marrow Transplant. 1995. 15:853–858.
4. Choi KH, Sung HJ, Lee WH, Kim HO, Lyu CJ, Min YH. Red cell depletion from the bone marrow aspirates for the ABO incompatible transplantation by apheresis separations. Korean J Hematol. 2001. 36:318–323.
5. Blacklock HA, Gilmore MJ, Prentice HG, Hazlehurst GR, Evans JP, Ma DD, Knight CB, Hoffbrand AV. ABO-incompatible bone-marrow transplantation: removal of red blood cells from donor marrow avoiding recipient antibody depletion. Lancet. 1982. 2:1061–1064.

6. Tichelli A, Gratwohl A, Wenger R, Osterwalder B, Nissen C, Burri HP, Speck B. ABO-incompatible bone marrow transplantation: in vivo adsorption, an old forgotten method. Transplant Proc. 1987. 19:4632–4637.
7. Benjamin RJ, McGurk S, Ralston MS, Churchill WH, Antin JH. ABO incompatibility as an adverse risk factor for survival after allogeneic bone marrow transplantation. Transfusion. 1999. 39:179–187.

8. Worel N, Greinix HT, Schneider B, Kurz M, Rabitsch W, Knobl P, Reiter E, Derfler K, Fischer G, Hinterberger W, Hocker P, Kalhs P. Regeneration of erythropoiesis after related- and unrelated-donor BMT or peripheral blood HPC transplantation: a major ABO mismatch means problems. Transfusion. 2000. 40:543–550.

9. Piomelli S, Karpatkin MH, Arzanian M, Zamani M, Becker MH, Geneiser N, Danoff SJ, Kuhns WJ. Hypertransfusion regimen in patients with Cooley's anemia. Ann N Y Acad Sci. 1974. 232:186–192.

10. Mielcarek M, Torok-Storb B, Storb R. ABO incompatibility and relapse risk in patients undergoing allogeneic marrow transplantation for acute myeloid leukemia. Bone Marrow Transplant. 2002. 30:547–548.

11. Goldman J, Liesveld J, Nichols D, Heal J, Blumberg N. ABO incompatibility between donor and recipient and clinical outcomes in allogeneic stem cell transplantation. Leuk Res. 2003. 27:489–491.

12. Worel N, Kalhs P, Keil F, Prinz E, Moser K, Schulenburg A, Mitterbauer M, Mannhalter C, Mayr WR, Schwarzinger I, Hocker P, Lechner K, Greinix HT. ABO mismatch increases transplant-related morbidity and mortality in patients given nonmyeloablative allogeneic HPC transplantation. Transfusion. 2003. 43:1153–1161.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download