Abstract
Astroblastoma is one of the very unusual type of tumors, whose histogenesis has not been clarified. It occurs mainly among children or young adults. Astroblastoma is grossly well-demarcated, and shows histologically characteristic perivascular pseudorosettes with frequent vascular hyalinization. Perivascular pseudorosettes in astroblastoma have short and thick cytoplasmic processes and blunt-ended foot plates. A 15-yr-old girl presented with headache and diplopia for one and a half year. A well-demarcated mass, 9.7 cm in diameter, was found in the right frontal lobe in brain MRI, and it was a well-enhanced inhomogenous mass. Cystic changes of various sizes were observed inside the tumor mass as well as in the posterior part of the mass, but no peritumoral edema was found. Histologically, this mass belongs to a typical astroblastoma, and no sign of anaplastic astrocytoma, gemistocytic astrocytoma or glioblastoma was found in any part of the tumor. Immunohistochemically, the tumor cells showed diffuse strong positivity for glial fibrillary acidic protein, S-100 protein, vimentin and neuron specific enolase, and focal positivity for epithelial membrane antigen and CAM 5.2, while showing negativity for synaptophysin, neurofilament protein, pan-cytokeratin and high molecular weight keratin.
Astroblastoma is a rare glial tumor occurring predominantly in the cerebral hemispheres of young adults. There have been numerous questions about the histogenesis of the astroblastoma (1). It is difficult to determine exact number of astroblastoma cases since the number may include some that are not typical astroblastoma. However in some report it was estimated that only 0.45-2.8% of all neuroglial tumors were astroblastoma (2). Domestically, no such cases have been reported so far. Astroblastomas are well demarcated and show characteristic perivascular pseudorosettes. Similar perivascular pseudorosettes may also occur partly in gemistocytic astrocytoma, anaplastic oligodendroglioma, anaplastic astrocytoma, glioblastoma, etc. Therefore, the term, astroblastoma, must be used only for the cases in which typical histological findings are observed throughout the tumor (3).
A 15-yr-old female patient had presented with headache and diplopia for one year and six months, and her headache in the area of right frontal lobe had become worse for the last month. The neurological examination showed diplopia, right nasal hemianopia, and papillary edema. Brain MRI showed a well demarcated mass, 9.7 cm in diameter, in right frontal lobe. After an injection of gadolinium, the tumor showed an inhomogenous enhancement. Within the tumor there were many cystic changes of different sizes (Fig. 1). It was completely removed surgically.
Histologically, perivascular pseudorosettes were observed throughout the tumor. In perivascular pseudorosettes, tumor cells were aligned along the fibrovascular stalk by one or two cell layers, but in some areas it was multilayered. Perivascular pseudorosettes became prominent in areas where the tumor cells were separated by artifacts (Fig. 2). Tumor cells forming perivascular pseudorosettes extended eosinophilic cytoplasmic processes toward vessel wall. Cytoplasmic processes were short and thick, of which blunt footplates were attached to the vessels (Fig. 3). Most tumor cells showed nuclear monotony with less atypism, yet a small number of them showed mild nuclear atypia. Multinucleated cells were occasionally observed in the areas with atypical nuclei. The tumor cell nuclei showed coarse chromatin pattern and no prominent nucleoli. Mitotic figures were rarely observed. Blood vessels were mostly of capillaries without smooth muscle layers. There was no glial fibrillarity in the fibrovascular stalk. The macrophages were frequently infiltrated in fibrovascular stalks. Vascular hyalinization or sclerosis was observed in some areas, but no endothelial proliferation was found (Fig. 4). Focal tumor necrosis was present. Areas of anaplastic astrocytoma, gemistocytic astrocytoma, and glioblastoma were not found in any part of the tumor.
Cytoplasmic processes of tumor cells composing perivascular pseudorosettes showed strong positive reaction for glial fibrillary acidic protein (GFAP) (Fig. 5). Tumor cells in discohesive areas showed GFAP-positive short cytoplasmic processes. The tumor cells showed diffuse strong positivity for S-100 protein, vimentin and neuron specific enolase (NSE), and focal positivity for epithelial membrane antigen (EMA) and CAM 5.2 (Fig. 6). The tumor cells were negative for synaptophysin, neurofilament protein (NFP), pan-cytokeratin, high molecular weight keratin (HMWK) immunostains. MIB-1 labeling index accounted for 8.0% (83/1,000), and p53 positivity was 16% (159/1,000) (Table 1).
Six weeks after the operation, radiation therapy of 4,500 cGy was performed for a period of seven weeks. Three years and nine months after the operation visual acuity decreased slowly and the left hemiplegia developed progressively. Multiple low intensity lesions was observed in T1-weighted image, and they were considered as radionecrosis. Four years after the operation the patient died of brain stem failure.
Since Bailey and Bucy reported on astroblastoma for the first time in 1930, the existence of the tumor itself has been one of the most interesting subjects (4). However, from the middle of the 1980s up until today, astroblastoma has been reported as a distinct entity (1, 5-9). Astroblastoma occur most frequently in young adults, occasionally in children. Congenital astroblastoma rarely occurred (2). Approximately 40 cases were reported in the literatures so far. The patients ranged from 1 to 58 yr old (average, 16 yr) (1, 8, 9). There seems to be no significant differences between genders (10, 11). Astroblastoma develops usually in cerebral hemisphere, but it can develop in the corpus callosum, cerebellum, optic nerve, brain stem, or in filum terminale (7, 11). In cerebral hemispheres, astroblastoma usually develops in cortex, subcortical area, and periventricular area, but not in the ventricle itself (11).
Astroblastoma is a well-demarcated tumor with variable sized cystic changes. Histologically, astroblastoma has characteristic perivascular pseudorosettes. Different from the perivascular pseudorosettes in ependymoma, those in astroblastoma have short and thick cytoplasmic processes. The cytoplasmic processes have blunt-ended footplates and are attached to basal lamina of blood vessels. In less cellular areas, perivascular pseudorosettes look more distinct. Typical perivascular pseudorosettes are observed even in the areas where tumor cells show solid growth patterns. Most of tumor cells have monotonous nuclei with coarse chromatin pattern. The blood vessels frequently show hyalinization and even sclerosis (1, 3, 8, 10, 11). This case showed all of these histological findings. Astroblastomas are classified into low grade and high grade according to histological findings (8). The high grade astroblastomas show increased cellularity with multiple cell layers upon the vascular walls. Tumor cells have severe nuclear atypia with increased mitotic figures. Vascular endothelial proliferation is also observed without prominent vascular hyalinization (8, 9). Overall histological findings in this case were compatible with the low grade astroblastoma.
Immunohistochemically, the tumor cells showed positivity for GFAP, S-100 protein, vimentin, NSE and EMA coinciding with the findings in other reports (2, 6, 12-14). The fact that neoplastic astrocytes may be positive for NSE and EMA is well known (12). However, reactions to CAM 5.2 reported as negative (2, 11, 12) or partly positive (13). This case showed positivity for CAM 5.2. Reason for the difference would be due to difference in antibodies used.
The term 'astroblastoma' is misleading in itself, since this tumor is not overtly astrocytic, nor are they 'blastic' (1). Bailey and Bucy believed that astroblastoma originated from astroblast, an intermediate stage between glioblasts and astrocytes (4). However, in a study by means of electron microscope it was proved that tumor cells of astroblastoma are intermediate between astrocytes and ependymal cells, and thus a possibility of tanycytes is presented as the origin of astroblastoma (7, 13, 15).
Tumors showing histological findings similar to astroblastoma are ependymoma, papillary meningioma, choroid plexus tumor, etc. Ependymoma is usually a well-demarcated mass and forms perivascular pseudorosettes, which may be confused with astroblastoma. It also shows positivity for GFAP, S-100 protein and vimentin and even focal positivity for cytokeratin, as astroblastoma does. However, perivascular pseudorosettes in ependymoma have thin and long cytoplasmic processes with no foot plates, which are different from the pseudorosettes of astroblastoma. And anuclear fibrillary zone is well developed around the blood vessels. Tumor cells between perivascular pseudorosettes are compact and show small ovoid monotonous nuclei with fine chromatin pattern. Papillary meningioma has perivascular papillary or pesudopapillary pattern with structures similar to pseudorosettes of ependymoma. The tumor cells of papillary meningioma show positive reaction for cytokeratin and negative reaction for GFAP. In addition, papillary meningiomas usually show connection to meninges as well as typical meningioma areas. Choroid plexus tumor also shows papillary growth pattern, which may be confused with astroblastoma. However, most of choroid plexus tumor develops within ventricle. Immunohistochemically choroid plexus tumor shows strong positivity for cytokeratin, while negativity or focal positivity for GFAP, and characteristic transthyretin positivity (16, 17).
The natural history of the astroblastoma seems to place it between the astrocytoma and glioblastoma (8). The low-grade astroblastomas are thought to have better prognosis than the high-grade astroblastomas. Gross total resection may result in long-term survival. Anaplastic histology has been associated with recurrence and progression, suggesting the more aggressive treatment, including radiotherapy, is necessary for high-grade lesions (1, 8, 9, 16).
Figures and Tables
Fig. 1
(A) T1-weighted image shows a huge well-demarcated mass in the right frontal lobe. (B) Many cystic changes of different sizes are observed within the tumor on T2-weighted image. (C) The tumor shows an inhomogenous enhancement.
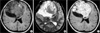
Fig. 3
The tumor cells composing perivascular pseudorosettes display short and thick cytoplasmic processes with prominent blunt-ended footplates toward the vessel wall (H&E, ×400).

Table 1
Immunohistochemical results and antibodies used in the study

-, negative; +, positive in less than 10% of tumor cells; ++, moderate to strong positive in 10-50% of tumor cells; +++, moderate to strong positive in more than 50% of tumor cells. GFAP, glial fibrillary acidic protein; NSE, neuron specific enolase; NFP, neurofilament protein; EMA, epithelial membrane antigen; pan-CK, pan-cytokeratin; LMWK, low molecular weight keratin; HMWK, high molecular weight keratin; LI, labeling index.
References
1. Brat DJ, Hirose Y, Cohen KJ, Feuerstein BG, Burger PC. Astroblastoma: Clinicopathologic features and chromosomal abnormalities defined by comparative genomic hybridization. Brain Pathol. 2000. 10:342–352.

2. Pizer BL, Moss T, Oakhill A, Webb D, Coakham HB. Congenital astroblastoma: an immunohistochemical study. Case report. J Neurosurg. 1995. 83:550–555.
3. Lantos PL, Rosenblum MK. Kleihues P, Cavenee WK, editors. Astroblastoma. Pathology and genetics of tumors of the nervous system. 2000. 2nd ed. Lyon: International Agency for Research on Cancer;88–89.
4. Bailey P, Bucy PC. Astroblastomas of the brain. Acta Psychiatr Neurol. 1930. 439–461.
5. Yamashita J, Handa H, Yamagami T, Haebara H. Astroblastoma of pure type. Surg Neurol. 1985. 24:218–222.

6. Hoag G, Sima AA, Rozdilsky B. Astroblastoma revisited: a report of three cases. Acta Neuropathol (Berl). 1986. 70:10–16.

7. Husain AN, Leestma JE. Cerebral astroblastoma: immunohistochemical and ultrastructural features. Case report. J Neurosurg. 1986. 64:657–661.
8. Bonnin JM, Rubinstein LJ. Astroblastomas: A pathological study of 23 tumors, with a postoperative follow-up in 13 patients. Neursurgery. 1989. 25:6–13.

9. Thiessen B, Finlay J, Kulkarni R, Rosenblum MK. Astroblastoma: Does histology predict biologic behavior? J Neurooncol. 1998. 40:59–65.
10. Burger PC, Scheithauer BW. Tumors of the central nervous system. Atlas of tumor Pathology. 1994. 3rd series. Washington DC: Armed Forces Institute of Pathology;146–148.
11. McLendon RE, Enterline DS, Tien RD, Thorstad WL, Bruner JM. Bigner DD, McLendon RE, Bruner JM, editors. Astroblastomas. Russell & Rubinstein's pathology of tumors of the nervous system. 1998. 6th ed. London: Arnold;419–426.
12. Cabello A, Madero S, Castresana A, Diaz-Lobato R. Astroblastoma: Electron microscopy and immunohistochemical findings: Case report. Surg Neurol. 1991. 35:116–121.

13. Jay V, Edwards V, Squire J, Rutka J. Astroblastoma: Report of a case with ultrastructural, cell kinetic, and cytogenetic analysis. Pediatr Pathol. 1993. 13:323–332.

14. Brat DJ, Cohen KJ, Sanders JM, Geuerstein BG, Burger PC. Clinicopathologic features of astroblastoma.[Abstract]. J Neuropathol Exp Neurol. 1999. 58:509.
15. Rubinstein LJ, Herman MM. The astroblastoma and its possible cytogenetic relationship to the tanycyte. Acta Neuropathol. 1989. 78:472–483.
16. Lantos PL, Vendenberg SR, Kleihues P. Graham DI, Lantos PL, editors. Tumors of the nervous system. Greenfield's neuropathology. 1997. 6th ed. London: Arnold;647–652.
17. Shim YR, Gu MJ, Kim DS, Kim OL, Byun WM, Kim YJ. Choroid plexus carcinoma. A report of two cases. Korean J Pathol. 2001. 35:176–179.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download