Abstract
This study was performed to investigate polymerase chain reaction-based detection of bacterial DNA in middle ear fluid and assess the correlation between the PCR-positive rate with several factors associated with middle ear effusion. The purpose was to gain a further understanding of bacterial infection as a major cause of otitis media with effusion. Of the 278 specimens of middle ear fluid, 39 (14%) tested positive by ordinary culture. The overall detection rate of bacterial DNA using the PCR method was 36.7% for middle ear effusion, and bacterial DNA detection rates of Hemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis in the middle ear effusion were 29.1%, 4.7% and 10.8%, respectively. The bacterial DNA detection rate was higher in ears with a history of acute otitis media than those without the history. High detection rates were observed in patients younger than 48 months who have had a higher tendency to present with acute otitis media. We concluded that PCR is a more sensitive method for the detection of bacteria in middle ear effusion than ordinary culture, and acute otitis media is a major contributor to the pathogenesis of otitis media with effusion.
Otitis media with effusion (OME) is the most common cause of hearing loss in children and requires antibiotic and surgical management. It can lead to adverse effects on speech and language development in children due to hearing disturbances if maintained for a long period of time (1). The exact mechanism of the pathogenesis of OME is not clearly understood. However, bacterial infection, Eustachian tube dysfunction, allergy and immunologic factors are known as major causes. Sinusitis and adenoid hypertrophy are also known to be involved (2). Since 1958 when Senturia et al. (3) reported the detection of bacteria in middle ear effusion, Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis have commonly been identified in numerous studies. This has resulted in regarding bacterial infection as an important factor in the pathogenesis of OME. The introduction of polymerase chain reactions (PCR) has resulted in a highly increased sensitivity and specificity of bacterial detection in middle ear effusion (4-9). Furthermore, studies based on the importance of bacterial infection in the pathogenesis of OME have become much more convenient.
In this study, the rate of bacterial infection using PCR was determined in middle ear effusion. The difference in the rate of bacterial detection in effusion was compared, followed by whether or not there was a history of acute otitis media and other factors involved in the pathogenesis of OME. Finally, the influence of bacterial infection in the development of OME was examined.
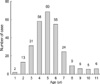
This study is based on 278 ears of 178 patients who visited the Department of Otolaryngology of the Hanyang University Hospital with otitis media with effusion from November 2000 to October 2002. These patients had undergone unsuccessful medical treatment, thereby maintaining the disorder for over 3 months. The age distribution was from 1 to 11 yr old and the average age was 5.1 yr old (Fig. 1).
The external auditory canal was cleansed with 70% alcohol for 1 min and a myringotomy was conducted. Middle ear fluid was then extracted using a Juhn tymp-tap (Xomed Treace Products, Jacksonville, FL, U.S.A.). A portion of the extracted middle ear fluid was inoculated for ordinary bacterial culture and the remaining was stored in -70℃ for PCR. For the ordinary bacterial culture, chocolate agar media was used for the Hemophilus influenzae culture, sheep's blood agar media and chocolate media for the Streptococcus pneumoniae culture and blood agar media and chocolate media for the Moraxella catarrhalis culture.
The genomic DNA was extracted by mixing 50 µL of the stored middle ear effusion with 900 µL cell lysis solution, followed by a 10 min centrifugation at 15,000 rpm at room temperature. DNA was extracted using the WIZARD Genomic DNA purification kit (Promega, Madison, WI, U.S.A.).
For the purpose of PCR, P6 protein was used as a primer for Hemophilus influenzae, PBP 2B for Streptococcus pneumoniae and M46 clone for Moraxella catarrhalis (Table 1) (4). 35 cycles of denaturation at 95℃, annealing at 55℃ and extension at 70℃ was carried out using a DNA thermal cycler. Electrophoresis was used for 30 min in 2% agarose gel for the detection of amplified product. Specimens of this study with consistent PCR results were used as positive controls and distilled water for a negative control.
Medical records of the subjects were examined for age, history of acute otitis media, history of ventilation tube insertion due to OME, characteristics of middle ear effusion, accompaniment of sinusitis and adenoid hypertrophy, etc. The PCR-positive rate was compared. History of acute otitis media was compared and categorized into two groups: a group of ears which have had acute otitis media within 6 months or have a history of experiencing acute otitis media more than 4 times a year, and a group of ears with no history of acute otitis media.
The difference in the detection rate was analyzed using the chi-square method (SPSS version 10.0) and the statistically significant rate was set at 95% and above (p<0.05).
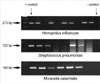
Bacteria were detected in 39 (14%) of 278 ears using ordinary bacterial culture. Hemophilus influenzae was detected in 22 cases (7.9%), Streptococcus pneumoniae in 4 cases (1.4%) and Moraxella catarrhalis was not detected at all. Other bacteria such as Staphylococcus aureus or Streptococcus pyogens were detected in 13 cases (4.7%). Using the PCR method, bacterial DNA was detected in 102 out of 278 cases (36.7%). Each detected bacteria was classified as follows: Hemophilus influenzae in 81 cases (29.1%), Streptococcus pneumoniae in 13 cases (4.7%) and Moraxella catarrhalis in 30 cases (10.8%) (Table 2) (Fig. 2).
Classifying the cases into two groups based on age, the positive rate of the group under 48 months (46.2% of 104 cases) was significantly higher than the positive rate of those over 48 months (31% of 174, p=0.013). When dividing the cases based on a previous history of acute otitis media, the positive rate of those with the history (44.5% of 110 cases) was significantly higher than those without the history (31.5% of 168 cases, p=0.031).
There was no significant difference between the positive rates of ears with a history of ventilation tube insertion and in ears diagnosed with OME for the first time. The detection rate based on the characteristics of middle ear effusion did not show a significant difference. The groups with accompanied sinusitis or adenoid hypertrophy did not show a significant difference from the groups without them (Table 3).
OME is a common disorder in children where effusion is contained in the middle ear cavity. Bacterial infection has been known as an important factor in the pathogenesis of OME since Senturia et al. (3) reported the detection of bacteria in middle ear effusion in 1958. Since then, Hemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been reported as the most common bacterial pathogens in numerous studies (10-12). However, the detection rate of these three bacteria in ordinary bacterial culture has been reported as follows: 8-20%, 4-10% and 2-8%, respectively (4). Three possible causes for these low positive rates are 1) the prolonged use of antibiotics before ventilation tube insertion will slow down the proliferation of pathogenic bacteria, 2) secretory immunoglobulin and lysozyme in middle ear effusion inhibits the proliferation of pathogenic bacteria, or 3) the bacteria in middle ear effusion exists as a biofilm (1, 13-15). However, high detection rates of 77.3% to 94.5% in middle ear effusion using PCR have been reported with a slight difference depending on the researcher in cases where bacteria were not cultured at all (14, 15). Choi et al. (12) and Park et al. (4) also reported a detection rate of 61.5% and 57.7%, respectively, in Korea in 1998 and 2000. In this study, bacteria was detected in only 14% of the cases using ordinary bacterial culture while bacterial DNA was detected in 36.7% using PCR. This bacterial detection rate is low compared to previous studies and this is presumed to be the result of a prolonged use of antibiotics before surgery and a lower contributing weight of bacterial infection than other factors in the pathogenesis of OME compared to the past.
There is still considerable controversy on whether or not the bacterial DNA detected by PCR implies the existence of metabolically active bacteria. However, in a study using a chinchilla model based on purified genomic DNA or pasteurized bacteria, it has been confirmed that neither were detected in middle ear effusion after 3 days while they were detected for over 21 days in cases where antibiotics were used for treatment (14). The mRNA of the GAPDH enzyme essential for bacterial DNA duplication or protein synthesis of metabolically active bacteria was detected by reverse transcriptase-polymerase chain reaction and proved that bacteria with mRNA had metabolic activity (1). However, whether or not the detected bacterial DNA implies acute infection of the middle ear has not yet been fully studied. The fact that the detection rate was high in ears with a history of acute otitis media implies that the remaining bacteria due to an insufficient use of antibiotics may be the cause of OME and that bacterial infection plays an important role in the progression of acute otitis media to OME. This is an important factor that must be considered while treating OME in children.
The fact that the detection rate was significantly higher in patients in the 48-month and younger age group, coupled with the fact that the frequency of acute otitis media is also higher in this age group, implies that bacterial infection is an important cause of OME.
Suzuki et al. (16), reported that the size of the adenoid and OME do not have any correlation, however, chronic infection of the adenoid acts as a reservoir of Hemophilus influenzae which is a major cause of OME. Furthermore, the possibility exists that Eustachian tube dysfunction is caused by adenoid hypertrophy. Mills et al. (17) reported that accompanied sinusitis plays an important role in the progression of OME by Eustachian tube dysfunction due to Eustachian tube salphingitis rather than direct bacterial dissemination. Therefore, obviously a simple comparison of bacterial-positive rates cannot definitively conclude a correlation of direct bacterial dissemination into the middle ear cavity by adenoid hypertrophy and sinusitis and OME. Qvarnberg et al. (11) stated that the bacteria-positive rate was higher in middle ear effusions with purulent fluid, however, there was no significant difference between those with mucoid and serous fluid. In this study, we presume that since the serous fluid specimens were much fewer than the mucoid fluid specimens, there were no statistically significant results and further study will be necessary. Allergy, known to be an important pathogenesis of OME, was excluded from this study and we believe that further research on this subject will be necessary.
Figures and Tables
Table 1
Primers used in polymerase chain reaction to detect major pathogenic bacteria in otitis media with effusion

References
1. Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998. 279:296–299.

2. Liu YS, Lim DJ, Lang RW, Brick HG. Chronic middle ear effusions: Immunochemical and bacteriological investigations. Arch Otolaryngol. 1975. 101:278–286.

3. Senturia BH, Gessert CF, Carr CD, Baumann ES. Studies concerned with tubotympanitis. Ann Otol Rhinol Laryngol. 1958. 67:440–467.
4. Park HJ, Park KH, Kim BC, Yoo YJ, Park K. Detection of bacteria in the middle ear effusion and adenoid tissue of chronic otitis media patient using PCR method. Korean J Otolaryngol. 2000. 43:913–917.
5. Jero J, Virolainen A, Salo P, Leinonen M, Eskola J, Karma P. PCR assay for detecting Streptococcus pneumoniae in the middle ear of children with otitis media with effusion. Acta Otolaryngol (Stockh). 1996. 116:288–292.

6. Ueyama T, Kurono Y, Shirabe K, Takeshita M, Mogi G. High incidence of Haemophilus influenzae in nasopharyngeal secretions and middle ear effusions as detected by PCR. J Clin Microbiol. 1995. 33:1835–1838.

7. Virolainen A, Salo P, Jero J, Karma P, Eskola J, Leinonen M. Comparison of PCR assay bacterial culture for detecting Streptococcus pneumoniae in middle ear fluid of children with acute otitis media. J Clin Microbiol. 1994. 32:2667–2670.
8. Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988. 239:487–491.

9. Bluestone CD. Recent advances in the pathogenesis, diagnosis and management of otitis media. Pediatr Clin North Am. 1981. 28:727–755.

10. Lim DJ, Lewis DM, Schram JL, Birk HG. Otitis media with effusion: Cytological and microbiological correlates. Arch Otolaryngol. 1979. 105:404–412.

11. Qvarnberg Y, Holopainen E, Palva T. Aspiration cytology in acute otitis media. Acta Otolaryngol (Stockh). 1984. 97:443–449.

12. Choi YC, Park YS, Yeo SW, Chae SY, Jung DG, Kim SW. Detection of Haemophilus influenzae and Streptococcus pneumoniae by polymerase chain reaction (PCR) in chronic otitis media with effusion (OME). Korean J Otolaryngol. 1998. 41:846–850.
13. Post JC, Aul JJ, White GJ, Wadowsky RM, Zavoral T, Tabari R, Kerber B, Doyle WJ, Ehrlich GD. PCR-based detection of bacterial DNA after antimicrobial treatment is indicative of persistent, viable bacteria in the chinchilla model of otitis media. Am J Otolaryngol. 1996. 17:106–111.

14. Post JC, Preston RA, Aul JJ, Larkins-Pettigrew M, Rydquist-White J, Anderson KW, Wadowsky RM, Reagan DR, Walker ES, Kingsley LA. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA. 1995. 273:1598–1604.

15. Gok U, Bulut Y, Keles E, Yalcin S, Doymaz MZ. Bacteriological and PCR analysis of clinical material aspirated from otitis media with effusions. Int J Pediatr Otorhinolaryngol. 2001. 60:49–54.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download