Abstract
The study of microsatellite instability (MSI) has provided the evidence to support asequential, progressive pathway for the development of cancer. In this study, we analyzed the role of MSI at chromosome 11p15.5 using microdissection of paraffin-embedded tissue from 68 matched normal and breast tumor samples. Components of intraductal, invasive and metastatic foci in lymph node were assessed for MSI using the polymorphic markers D11S922, tyrosine hydroxylase (TH) and D11S988. We found that MSI at D11S922 was relatively high incidence than other two markers and increased during breast cancer progression. The overall frequency of MSI at D11S922 was 26.7% in pure intraductal carcinoma, 36.4% in invasive carcinoma, and 40.0% in invasive carcinoma with metastases. We observed no significant correlation between MSI at chromosome 11p15.5 and the patient's age, tumor size, histological grade, or lymph node metastasis. We compared the MSI incidence with the expression of prognostic markers, such as p53, c-erb B2, estrogen receptor, and progesterone receptor, and found no significant correlation. We suggest that the MSI of chromosome 11p15.5 is increased during breast cancer progression, but long-term follow-up study would establish whether MSI at chromosome 11p15.5 could be useful as a potential prognostic marker for breast cancer.
Breast cancer is the most common malignant tumor and has the second highest mortality rate in western women (1). Recently in Korea, the incidence of breast cancer has increased along with an increase in western lifestyle. The majority of cases appear to be sporadic, but hereditary factors may be involved in about 5-10% of all cases (2). Extensive research into the factors influencing development and prognosis of breast cancer has been carried out, but the translation of these risk factors to clinical utility has been limited.
In general, the activation of oncogenes and inactivation of tumor suppressor genes underlie carcinogenesis, and tumors develop through an accumulation of several genetic alterations (3). Recently, molecular studies have provided the evidence to explain the development and progression of cancer at the cellular level. Loss of heterozygosity (LOH) is a change from a heterozygous to a homozygous state due to loss of the wild type allele, and is important in the identification of new tumor suppressor genes (4). Colon cancer represents a good model of cancer progression from adenoma to carcinoma that is accompanied by genetic mutations and LOH (5). However, breast cancer is morphologically and biologically heterogeneous, so it has been difficult to define a general series of genetic aberrations involved in progression of this cancer type.
Many studies have revealed that LOH at chromosome bands 1p36, 3p24-p25, 6q12-q16, 11p15, 11q22, 11q23, 13q21, 16q22, 17p21, and 17q25, plays important roles in breast cancer, and these genetic alterations have complex interactions (6-12). In particular, alterations of both the long and short arms of chromosome 11 are involved in the progression of breast cancer, and loci at 11p15.5, 11q13.1, and 11q23.3 are importantly considered (10, 13-17). In lobular carcinoma, the most common region of LOH is at 11q13, and in ductal carcinoma loci at 11q23 and 11p15.5 are important (16). Phillips et al. determined the potential effects of chromosome 11 on the tumorigenic and metastatic abilities of the MDA-MB-435 cell line via microcell-mediated chromosome transfer, and indicated that human chromosome 11 harbors a metastasis-suppressor gene for human breast cancer (18).
Previous studies using fresh frozen tissue have often been problematic since the surrounding non-neoplastic cells (e.g., stromal and inflammatory cells) confound analysis of the tumor cell population. Microdissection of paraffin-embedded tissues allows the isolation of pure populations of neoplastic or non-neoplastic cells (20). Retrospective studies correlating histopathologic findings with genetic alterations become possible.
Until now, the association between chromosome 11p15.5 and various clinical parameters has not been well defined. Prognostic factors of breast cancer include expression of c-erb B2, p53, and the estrogen and progesterone receptors (21, 22). We isolated normal, intraductal carcinoma, invasive ductal carcinoma, and metastatic lesions in lymph nodes using microdissection technique on paraffin-embedded tissue, and assessed the incidence of microsatellite instability (MSI) at chromosome 11p15.5 in comparison with several clinical parameters and other prognostic factors, such as c-erb B2, p53, estrogen and progesterone receptors (21, 22).
Formalin-fixed and paraffin-embedded tissues of primary tumor and lymph nodes from primary breast cancer patients without distant metastasis were obtained upon surgical resection specimen at Kyungpook National University Hospital. The 68 cases were selected from 150 breast cancer cases between 1999 and 2000 on the basis of the availability of tumor and matched normal tissues. Tumors were examined for the presence of normal, intraductal, invasive and lymph node metastatic lesion and each component was isolated by microdissection technique.
Surgical specimens were fixed in 10% buffered formalin and embedded in paraffin and serial 4-5 µm tissue sections were stained with hematoxylin and eosin.
Serial tissue sections of 4 µm thickness from paraffin block were attached on poly-L-lysine (Sigma) coated slide. The slides were deparaffinized in xylene and carried through 2 changes of absolute ethanol. After the pretreatment in 10 mM citrate buffer (pH 6.0), the slides were subjected to microwave oven for 15 min. The endogenous peroxidase activity was blocked with 3% H2O2 for 15 min and the slides were washed with Tris-buffered saline (TBS, 0.1 M, pH 7.6) three times for 5 min. Nonspecific binding was blocked with 5% normal goat serum for 20 min. The antibodies used in this study include p53 (DO7, DAKO, Carpinteria, CA, U.S.A.), ER (ER1D5, Immunotech, U.S.A.), PR (clone 1Ab, Immunotech) with 1:50 dilution and c-erb B2 (A0485, DAKO) with 1:100 dilution. Diluted primary antibodies were applied, followed by incubation at room temperature for 1 hr and washed with TBS three times. Sections were incubated with biotinylated antibody (LSAB Kit, DAKO) for 35 min, followed by streptavidin-biotin complex (LSAB Kit, DAKO). The antigen-antibody binding was visualized with diaminobenzidine and the sections were counterstained with hematoxylin. The criteria for interpretation of immunohistochemical staining are below. No staining or weak staining was classified as negative. For p53, ER, and PR, the case showing more than 10% strong nuclear staining was regarded as positive. For c-erb B2, a strong cytoplasmic membrane staining pattern was regarded as positive.
Unstained 10 µm tissue sections on the glass slides were deparaffinized with xylene and rinsed twice with ethanol. The slides were stained with toluidine blue and air dried. The target slides were rinsed in a 2% glycerol in TE buffer and the 32 gauge needle tip was placed onto the target cell under ×100 magnification. Areas of intraductal, invasive carcinoma and metastatic lesion in lymph nodes were dissected under microscopic observation. After the completion of dissection, the micromanipulator was pulled and the tip of needle was soaked in a 0.5 mL microcentrifuge tube containing 20 µL of DNA extraction buffer (0.5 µg of proteinase K in 1 µL of 1% Tween 20 in TE buffer). Repeating this procedure, 100-200 numbers of cells were obtained and at least 5-10 numbers of cells were present per 1 µL. The mixtures of cells with DNA extraction buffer were incubated at 52℃ for 1-2 days and boiled to inactivate proteinase K for 10 min at 100℃. The genomic DNA of microdissected samples were purified using QIAamp DNA mini kit (Qiagen, Germany) and quantified using spectrophotometer. We used 200 ng of the genomic DNA as a template for PCR of microsatellite analysis.
Each microsatellite was amplified by the polymerase chain reaction (PCR) using GeneAmp PCR reagent kit 9600 (Perkin Elmer Cetus, Norwalk, CT, U.S.A.), 10×PCR buffer 1 µL, 25 mM MgCl2 0.6 µL, 1.25 mM dNTP mixture 1.6 µL, 25 pM each primer 0.4 µL, Taq DNA polymerase 0.5 Unit and DNA template 200 ng, in total volume of 10 µL. Cycling conditions consisted of 30 cycles at 94℃, 60 and 72℃ for 30 sec, with final extension at 72℃ for 10 min. After PCR, the products were confirmed by electrophoresis in 1.8% agarose gels and stained with 0.5 g/mL ethidium bromide. For analysis of microsatellite instability, the PCR products were electrophoresed in 8% acrylamide gel containing 7 M ureas and visualized by silver staining. Tumors were considered as having MSI when the tested microsatellite loci showed a different banding pattern and a significant difference in relative intensity of the bands compared to the corresponding normal counterpart.
The clinical data of 68 breast carcinoma patients are listed in Table 1. There were 15 cases of pure intraductal carcinoma (DCIS) and 53 cases of invasive ductal carcinoma (IDC). All patients were female and the mean age was 48 yr. Twenty of these 53 cases had lymph node metastases and two of those 20 cases had intraductal components. Among the 33 cases of invasive carcinoma without lymph node metastasis, 14 patients had coexisting intraductal components. Tumor size was classified into three groups; 17 cases of less than 2 cm, 22 cases of 2-5 cm, 14 cases of greater than 5 cm in greatest diameter.
Microscopically, the intraductal carcinomas were grouped by Van Nuys classification into four cases of group 1, five cases of group 2, and six cases of group 3. Invasive ductal carcinomas were also classified by Bloom and Richardson grading system into 14 cases of grade I, 24 cases of grade II, and 15 cases of grade III.
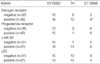
The overall incidences of MSI in the intraductal and invasive carcinoma were 26.7% and 45.3% at D11S922, 13.3% and 33.9% at TH, and 6.7% and 18.9% at D11S988 respectively (Table 2). Data from 41 cases that have MSI for at least one polymorphic marker are presented in Fig. 1. The patterns of MSI in 4 of 41 cases are shown in Fig. 2.
The MSI data from cases was analyzed by each tumor component. In 33 cases of IDC without metastasis, the incidence of MSI at D11S922, TH, and D11S988 were 36.4%, 27.3%, and 15.2% in invasive component and 18.7%, 18.7%, 12.5% in 14 cases of coexisting intraductal component. In 20 cases with lymph node metastases, it was 40%, 30%, and 25% in invasive component and 35.0%, 25.0%, 20.0% in lymph node metastatic lesion, respectively. The intraductal component in IDC was only 2 cases and has no significant result. The incidence of MSI increased through the subsequent stages of cancer progression but showed no significant difference in statistical analysis. MSI incidence in intraductal lesions coexisting with invasive carcinoma did not significantly increase the risk of progression to invasive carcinoma.
The MSI frequency at D11S922, TH, or D11S988 according to clinicopathological parameters such as the patient's age, tumor size, and lymph node metastasis are presented in Table 3 and no significant difference was observed.
The positivity rates of immunohistochemical stain in 68 cases were as follows: estrogen receptor positivity was 64.7%, progesterone receptor was 61.7%, p53 was 39.7%, and c-erb B2 was 54.4%. The comparisons between MSI at D11S922, receptor, p53, c-erb B2 are listed in Table 4. The correlation between MSI of D11S988 and expression of estrogen receptor was statistically significant (p=0.041).
Among the theories related to the development mechanism of breast cancer presented up to now, according to the theory of Newsham, when a normal ductal epithelium progresses to intraductal carcinoma through atypical proliferation, the locus of the genes of chromosomes 11p15.5 and 11q23 are lost and when it progresses to invasive carcinoma, chromosome 11q13 is lost (16). However, the role of 11p15.5 is not clearly identified yet. We used D11S922, TH, and D11S988 as the microsatellite markers that correspond to chromosome 11p15.5 in intraductal and invasive carcinoma and these parts correspond to the part of 6Mb that codes the IGF-II (insulin-like growth factor-II), the p57KIP2, and the KvLQT1 gene. The IGF-II is an important growth factor and the p57KIP2 is the cyclin-dependent-kinase inhibitor that provokes arrest of the G1-S stage (23). The KvLQT1 gene is involved in a voltage-gated potassium channel.
In this research, the MSI of the locus of the gene D11S922 was relatively higher than another markers, and was 26.7% in pure intraductal carcinoma, 36.4% in invasive ductal carcinoma without lymph node metastasis, and 40.0% in invasive ductal carcinoma with lymph node metastasis. It shows a high frequency according to the progression of breast cancer, but was not statistically significant. This shows a similar result to the other research using the existing paraffin embedded tissue. MSI incidences at TH and D11S999 loci were also increased during histologic progression, but the data cannot be interpreted as representative and significant because of limited positive results.
According to the research of Lichy et al., the LOH of 11p 15.5 is 37.5% in pure intraductal carcinoma, 32% in invasive ductal carcinoma without lymph node metastasis, and 44% in invasive ductal carcinoma with lymph node metastasis (24). Their result was also different from ours because it reported that the LOH of 11p15.5 is mainly involved when the normal epithelial cell progresses to intraductal carcinoma.
In the research of Winqvist et al., it is reported at 35% overall frequency (15). According to the report of Deng and others, the LOH of the chromosome 11p15.5 is also observed in the normal terminal duct lobular unit. This suggests that the LOH of chromosome 11p15.5 can play the role of a genetic shunt progressing toward the infiltrating carcinoma from normal epithelial tissues (25). That is to say, cancer may occur in another pathway and not in a series of continuous pathways of the normal epithelial cell, intraductal carcinoma, invasive carcinoma, and metastatic carcinoma. In the present research, we observed that the MSI of chromosome 11p15.5 has a lower frequency in intraductal carcinoma than the invasive carcinoma; however, the comparison of intraductal carcinoma by histology and grade has no significance probably due to the limitation of the number of cases. In the research of Fujii and others regarding the loss of genes according to the histologic grade of intraductal carcinoma, the number of lost genes increases significantly at a nuclear grade higher and in a lower grade, it was observed that 16q and 17p were lost (26). However, the loss of 11p is related to high-grade lesion and it is observed at around 40%. It is reported that intraductal carcinoma lesions accompanied with infiltrating cancer and pure intraductal carcinoma lesions are different from each other in the form of heterozygosity and it is considered that tumors have been developed from intraductal carcinoma lesions to infiltrating carcinoma through various genetic pathways (26).
In the present research, there is no statistical significance as a result of analyzing the correlation of the MSI of 11p15.5 with the patient's clinical factors such as age of onset, size of tumor, grade, and presence of lymph node metastasis. As for the correlation between the factors such as p53, c-erb B2, estrogen and progesterone receptor, which are known as the prognostic factors for breast cancer, the estrogen receptor showed a significant correlation with the MSI of D11S988. But it is necessary to evaluate the correlation to the patient's survival rate if the MSI of 11p15.5 could be useful as a potential prognostic marker for breast cancer.
As a result, we suggest that the MSI of chromosome 11p 15.5 could play a part in the occurrence and progression of breast cancer, because the more cancer progresses, the higher its frequency increases. But its role in the continuous progressive stage of breast cancer could not be clearly verified. It is considered necessary to study further the worth of which MSI in the chromosome 11p15.5 is used as the method to predict the prognosis of breast cancer.
Figures and Tables
Fig. 1
Pattern of microsatellite instability in 41 of 68 cases presenting at least one polymorphic marker. Invasive ductal carcinoma cases are from case number 1 to 52 and ductal carcinoma in situ cases are from case number 54 to 66. ● microsatellite instability, ○ retention of heterozygosity, ⊚ uninformative.

Fig. 2
Pattern of MSI at D11S922, D11S988 and TH. (A) Case no. 10 IDC with lymph node metastasis and coexisting DCIS (D11S 922). (B) Case no. 1 IDC with lymph node metastasis (D11S922). (C) Case no. 63 DCIS (D11S922). (D) Case no. 10 IDC with lymph node metastasis, coexisting DCIS (D11S988). (E) Case no. 28 IDC with lymph node metastasis (TH). N-normal, D-ductal carcinoma in situ, I-invasive ductal carcinoma, L-lymph node metastasis.
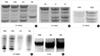
Table 2
Overall incidence of microsatellite instability at 11p15.5 loci in breast ductal carcinoma (%)

References
1. Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1997. 47:5–27.
2. Andersen TI. Genetic heterogeneity in breast cancer susceptibility. Acta Oncol. 1996. 35:407–410.

3. Niederacher D, Schnurch HG, An HX, Ellenberger I, Dall P, Van Roeyen CR, Kuppers V, Beckmann MW. Detection of sequential genetic alterations relevant for breast cancer development. Eur J Cancer Prev. 1996. 5:497–503.
4. Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell JE. Molecular cell biology. 4th ed. New York: W.H. Freeman and company;1063–1069.
6. Callahan R, Campbell G. Mutations in human breast cancer: an overview. J Natl Cancer Inst. 1989. 81:1780–1786.

7. Genuardi M, Tsihira H, Anderson DE, Saunders GF. Distal deletion of chromosome 1p in ductal carcinoma of the breast. Am J Hum Genet. 1989. 45:73–82.
8. Matsumoto S, Minobe K, Utada Y, Furukawa D, Onda M, Sakamoto G, Kasumi F, Nakamura Y, Emi M. Loss of heterozygosity at 3p24-p25 as a prognostic factor in breast cancer. Cancer Letters. 2000. 152:63–69.

9. Tomlinson IP, Nicolai H, Solomon E, Bodmer WF. The frequency and mechanism of loss of heterozygosity on chromosome 11q in breast cancer. J Pathol. 1996. 180:38–43.

10. Hampton GM, Mannermaa A, Winquist R, Alavaikko M, Blanco G, Taskinen PJ, Kiviniemi H, Newsham I, Cavenee WK, Evans GA. Loss of heterozygosity in sporadic human breast carcinoma: a common region between 11q22 and 11q23.3. Cancer Res. 1994. 54:4586–4589.
11. Van Den Berg J, Johannsson O, Hakansson S, Olsson H, Borg A. Allelic loss at chromosome 13q12-q13 is associated with poor prognosis in familial and sporadic breast cancer. Br J Cancer. 1996. 74:1615–1619.

12. Iida A, Isobe R, Yoshimoto M, Kasumi F, Nakamura Y, Emi M. Localization of a breast cancer tumour-suppressor gene to a 3-cM interval within chromosomal region 16q22. Br J Cancer. 1997. 75:264–267.

13. Ali IU, Lidereau R, Theillet C, Callahan R. Reduction to homozygosity of genes on chromosome 11 in human breast neoplasia. Science. 1987. 238:185–188.

14. Karnik P, Plummer S, Casey G, Myles J, Tubbs R, Crowe J, Williams BR. Microsatellite instability at a single locus (D11S988) on chromosome 11p15.5 as a late event in mammary tumorigenesis. Hum Mol Genet. 1995. 4:1889–1894.

15. Winqvist R, Hampton GM, Mannermaa A, Blanco G, Alavaikko M, Kiviniemi H, Taskinen PJ, Evans GA, Wright FA, Newsham I. Loss of heterozygosity for chromosome 11 in primary human breast tumors is associated with poor survival after metastasis. Cancer Res. 1995. 55:2660–2664.
17. Karnik P, Paris M, Williams BR, Casey G, Crowe J, Chen P. Two distinct tumor suppressor loci within chromosome 11p15 implicated in breast cancer progression and metastasis. Hum Mol Genet. 1998. 7:895–903.

18. Phillips KK, Welch DR, Miele ME, Lee JH, Wei LL, Weissman BE. Suppression of MDA-MB-435 breast carcinoma cell metastasis following the introduction of human chromosome 11. Cancer Res. 1996. 56:1222–1227.
19. Chen T, Dhingra K, Sahin A, Sneige N, Hortobagyi G, Aldaz CM. Technical approach for the study of the genetic evolution of breast cancer from paraffin-embedded tissue sections. Breast Cancer Res Treat. 1996. 39:177–185.

20. Lee JY, Dong SM, Kim SY, Yoo NJ, Lee SH, Park WS. A simple, precise and economical microdissection technique for analysis of genomic DNA from archival tissue sections. Virchows Arch. 1998. 433:305–309.

21. Youngson BJ, Anelli A, Van Zee KJ, Borgen PI, Norton L, Rosen PP. Microdissection and molecular genetic analysis of HER2/neu in breast carcinoma. Am J Surg Pathol. 1995. 19:1354–1358.

22. Dahiya R, Deng G. Molecular prognostic markers in breast cancer. Breast Cancer Res Treat. 1998. 52:185–200.

23. Feinberg AP. Imprinting of a genomic domain of 11p15 and loss of imprinting in cancer: an introduction. Cancer Res. 1999. 59:1743s–1746s.
24. Lichy JH, Zavar M, Tsai MM, O'Leary TJ, Taubenberger JK. Loss of heterozygosity on chromosome 11p15 during histological progression in microdissected ductal carcinoma of the breast. Am J Pathol. 1998. 153:271–278.

25. Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996. 274:2057–2059.

26. Fujii H, Szumel R, Marsh C, Zhou W, Gabrielson E. Genetic progression, histological grade and allelic loss in ductal carcinoma in situ of the breast. Cancer Res. 1996. 56:5260–5265.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download