Abstract
During the preclinical study of new therapeutic modality, we evaluate whether the treatment can reverse the established asthma phenotypes in animal model. However, few have reported on the long term persistence of asthma phenotypes upon re-challenge with allergen (secondary challenge) in animal model. We evaluated the persistence of asthma phenotypes by secondary challenge at different times in previously challenged murine asthma model. BALB/c mice sensitized by intraperitoneal injections of 20 µgram of ovalbumin and 1 mg of alum on days 1 and 14 were challenged initially by the inhalation of 1% ovalbumin for 30 min on days 21, 22, and 23. Each group of mice was rechallenged at 5, 7, 9, or 12 weeks after the initial challenge. Airway hyperresponsiveness, BAL fluid, airway histology and serum ovalbumin-specific IgE level were evaluated. Airway eosinophilia, airway inflammation and serum ovalbumin-specific IgE production persisted upon secondary allergen challenges at least 12 weeks after the initial challenge. However, airway hyperresponsiveness persisted only until mice were rechallenged 7 weeks after the initial challenge. Airway inflammation and allergen specific IgE production may persist longer than airway hyperresponsiveness in a mouse asthma model of secondary allergen challenge.
Allergic asthma is characterized by the intermediate phenotypes of chronic inflammation of airway with predominant eosinophilic infiltration, airway hyperresponsiveness, reversible airway obstruction, and allergen specific IgE production. The prevalence of asthma is increasing worldwide, also in Korea (1). Asthma reduces the quality of life and may result in mortality. It is regarded as one of most important public health problems. The morbidity and mortality associated with asthma are still increasing, especially during the last half 20th century, despite the currently available mediations, which demands new therapeutic modalities (2). Animal models are essential in the study of the mechanisms of allergic diseases and for the development of new therapies. The mouse is one of the most popular species in the field of asthma study. It has several advantages; a well-known immune system, the availability of many tools to manipulate the immunological process, the availability of numerous genetically modified (transgenic or knockout) strains, and cost.
The persistence of a disease phenotype is important in the evaluation of newly developed treatment modalities in animal models. During the preclinical study, we evaluate whether the treatment can reverse the established asthma phenotypes in animal model. Many reports have been issued on mouse models of asthma (3) but few have reported upon the long term persistence of asthma phenotypes upon secondary allergen challenge in previously sensitized and challenged mice. For these reasons, we undertook to evaluate the persistence of asthma phenotypes in a mouse model after secondary allergen challenge at different times after sensitization and initial challenge.
Seven weeks old BALB/c female mice were used in this study. The mice were purchased from Dae Han Biolink (Choongbuk, Korea) and kept in specific pathogen free conditions in the Preclinical Center of the Clinical Research Institute of Seoul National University Hospital (Seoul, Korea). None of the mice was exposed to ovalbumin (OVA) before the experiment. The study was approved by the Committee on Animal Experimentation at our institution and performed according to the guide for the care and use of laboratory animals issued by the Institute of Laboratory Animal Resources Commission on Life Sciences, National Research Council, U.S.A. (4).
The protocol used for sensitization and bronchial challenge was the same as previously described (5). There were five groups and each group consists of five mice; mice initially challenged, mice initially challenged and rechallenged at 5, 7, 9, or 12 weeks after the initally challenge (Fig. 1). Mice were sensitized by intraperitoneal injections of 20 µg of ovalbumin (OVA, ICN, Costa Mesa, U.S.A.) with 1 mg of alum hydroxide (alum, Sigma, St. Louis, U.S.A.) on days 1 and 14. The initial inhalation challenge was done with 1% OVA for 30 min daily on days 21, 22 and 23 with an ultrasonic nebulizer (NE-U12, Omron, Japan). Each group of mice were rechallenged at different times (5, 7, 9, or 12 weeks after the initial challenge) with 1% OVA for 30 min daily and for 3 consecutive days. Mice sensitized and challenged by PBS at each time were used as negative controls for possible environmental factors.
Airway hyperresponsiveness: Twenty-four hours after the final OVA inhalation, airway hyperresponsiveness was assessed by methacholine-induced airflow limitation using single chamber whole body plethysmography (Allmedicus, Anyang, Korea) as previously described (5, 6). Increases in enhanced pause (Penh) were measured as an index of airway resistance {Penh=[(Te/RT-1)×(PEF/PIF)], where Penh=enhanced pause, Te=expiratory time (s), RT=relaxation time (s), PEF=peak expiratory flow (mL/s), PIF=peak inspiratory flow (mL/s)}. Increasing doses of methacholine (ranging from 2.5 to 50 mg/mL; Sigma, St. Louis, U.S.A.) were administered by nebulization for 3 min, and the values of Penh were calculated over the following 3 min. During the experiment, the activity of the mice and the barometric plethysmograph flow tracings were monitored. For the quantification of the dose-response to methacholine, the linear regression of Penh on log was calculated for individual mice. The log dose corresponding to an increase in Penh of 200%, respectively, was determined, and the average log doses of the different groups were compared. The results are presented as PC200, which is the concentration of methacholine required to increase the baseline Penh by 200%.
Inflammatory cells in bronchoalveolar lavage (BAL) fluid: Forty-eight hours after the final OVA challenge, mice tracheae were cannulated and the lungs were lavaged five times with 0.4 mL aliquots of pyrogen-free saline. After Diff-quickR staining (Dade Behring AG, Dudingen, Switzerland) of lung lavage cells in a cytospin preparation, two investigators blindly counted more than 300 inflammatory cells under a light microscope and classified them as macrophages, lymphocytes, neutrophils, or eosinophils.
Lung histology: Following BAL, the lungs were infused with 10% formalin and embedded in paraffin. Lung sections were stained with hematoxylin and eosin. Slides were assessed by light microscopy and the degree of peribronchial and perivascular inflammation was evaluated on a subjective scale of 0-3, as previously described (5, 7). The investigators who scored airway inflammation were blinded as to which preparation they were scoring. Briefly, a value of 0 was assigned when no inflammation was detectable, a value of 1 for occasional cuffing with inflammatory cells, a value of 2 when most bronchi or vessels were surrounded by a thin layer (one to five cells) of inflammatory cells and a value of 3 when most bronchi or vessels were surrounded by a thick layer (more than five cells deep) of inflammatory cells. The total lung inflammation was defined as the average of the peribronchial and perivascular inflammation scores.
Serum ovalbumin specific IgE: Forty-eight hours after the final OVA challenge, blood samples were obtained from the mice via the inferior vena cava. Anti-OVA specific antibodies were measured by ELISA, as previously described (5). Briefly, microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with 2 µg/mL of OVA in a 50 mM of carbonate buffer (pH 9.6) at 4℃ Nonspecific binding was blocked with 2% bovine serum albumin for 1 hr at 20℃ After incubation of the test sera for 2 hr, the plates were incubated with horseradish peroxidase-labeled goat anti-mouse IgE (Pharmingen, San Diego, U.S.A.) for 1 hr at 20℃. The reaction was developed with a tetramethylbenzidine (Sigma, St. Louis, U.S.A.) and stopped by adding 2 N H2SO4. The optical density was measured at 490 nm, and the antibody titers of the samples were related to pooled standards, which were generated in the laboratory; results are expressed in arbitrary units (AU) according to each O.D. value.
Statistical analysis was performed using the Kruskal-Wallis and the Mann-Whitney U tests. Statistical significance was accepted at p<0.05. Analysis was performed using SPSS 9.0. Data are expressed as the means±standard error with the exception of the inflammatory scores, which are expressed as the means±standard deviation.
Airway hyperresponsiveness upon a secondary OVA challenge was persistent when mice were rechallenged 5 or 7 weeks after the initial inhalation challenge. The values of PC200 observed in the rechallenged animals at 5, or 7 weeks after the initial challenge were similar to that of initially challenged group (5.30±0.30, 10.6±3.50 vs. 7.37±3.63 mg/mL, p>0.05), but the values of PC200 at 9 and 12 weeks after the initial challenge were higher than that of initially challenged group (30.20±11.60, 19.5±15.2 vs. 7.37±3.63 mg/mL, p<0.05).
Mice sensentized and challenged with PBS showed constantly high values of PC200 from the initial challenge (41.30±8.67, 39.3±10.7, 33.48±10.2, 41.49±8.50, 45.20±4.80 mg/mL, p>0.05), which means that there was no environmal factor that may alter the physiological air flow during the experiment (Fig. 2).
The percentages of BALF eosinophils in the secondarily challenged mice were higher even in mice that were rechallenged 12 weeks after initial challenge. The proportions of BALF eosinophils observed in the rechallenged animals at 5, 7, 9, or 12 weeks after the initial challenge were similar to that of initially challenged group (37.8±9.7, 34.7±4.6, 29.4±9.1, 23.3±6.0 vs. 39.6±9.3 %, p>0.05). PBS treated mice showed no eosinophil in BAL fluid at initial challenge and even at the end of the study (0.0±0.0%). (Fig. 3A)
The degree of inflammation was evaluated in 10 random high power fields (×400). Peribronchial, perivascular and total inflammation scores in the rechallenged animals at 5, 7, 9, or 12 weeks were similar to that of initially challenged group (p>0.05). The inflammatory scores of PBS treated mice were lower than those of OVA treated mice (p<0.05) (Fig. 3B). Epithelial shedding was not found and the thickness of subepithelial fibrosis was similar in all groups.
The serum anti-OVA specific IgE level was still increased when mice were rechallenged 12 weeks after the initial challenge. The levels of serum anti-OVA specific IgE observed in the rechallenged animals at 5, 7, 9, or 12 weeks were not different to that of initially changed group (72.8±24.7, 18.0±5.6, 65.2±45.8, 21.5±5.0 vs. 50.2±12.1 A.U., p>0.05). We could not detect serum OVA-specific IgE production in PBS treated mice (0.0±0.0 A.U.) (Fig. 4).
In this study, we found that eosinophilic airway inflammation and the increased serum allergen-specific IgE production persisted upon a secondary challenge at least 12 weeks after the initial challenge in a murine model of asthma. Airway hyperresponsiveness, another characteristic of asthma, persisted when asthmatic mice were reexposed to allergen only 5 to 7 weeks after the initial challenge.
It is important to determine the duration of a persistent disease phenotype in an experimental protocol. The majority of previous investigations have examined the effects of therapeutic intervention at the time of sensitization or before the initiation of allergic responses in mouse models (5, 8-10). However, we see patients who have established asthma in a clinical situation. Therefore, we should be aware as to whether the therapeutic intervention can reverse the established asthma phenotypes. In this study, we evaluated the persistence of asthma phenotypes upon a secondary challenge in previously challenged murine asthma model.
The persistence of asthma phenotypes might vary according to the experimental protocols used, especially in terms of the types and dosages of allergens, adjuvants, and the strains of mice. We chose ovalbumin, alum, and BALB/c mice because they are one of the most popular systems used in asthma study (3). It has been reported that asthma phenotypes, including airway hyperresponsiveness persist for at least 7 weeks in a mouse model of asthma using ragweed allergen. However, the study used a different allergen and three ragweed challenges were performed during the experiment (11). Another study revealed that eosinophilic inflammation of airways persisted for 8 weeks in a mouse model of asthma using ovalbumin (10). Recently, Kanehiro et al. (12) rechallenged previously challenged animals 6 weeks after the initial challenge using ovalbumin, and measured airway resistance and dynamic compliance in anesthetized, mechanically ventilated mice. They found that airway hyperresponsiveness and eosinophilic inflammation of airway persisted.
We evaluated the persistence of asthma phenotypes over a longer period with different model system. Though our results concur with those of the previous reports in similar period (10-12), we found that airway hyperresponsiveness upon a secondary challenge disappeared when mice were reexposed to allergen 9 or 12 weeks after initial challenge. However, airway eosinophilia (IL-5 dependent) and serum allergen specific IgE production (IL-4 dependent) were found to be elevated when mice were rechallenged even 12 weeks after the initial challenge. This finding suggests that the airway inflammation, allergen-specific IgE production and airway hyperresponsiveness may be regulated by different mechanisms.
Airway hyperresponsiveness is a characteristic feature of asthma and is used as an index of the asthma phenotype in a mouse model (3). Airway inflammation, airway wall thickening, airway epithelial damage, edema of the airways, alteration of presynaptic control of acetylcholine release, activation of local axonal reflexes, altered airway smooth muscle function, or a combination of several of these events have been suggested as possible mechanisms of airway hyperresponsiveness (13). As for the cytokines, there was initial confusion about the relative importance of IL-4 and IL-5 in the pathogenesis of airway hyperresponsiveness (3). Anti-IL-4 antibody treatment reduced airway hyperresponsiveness in sensitized BALB/c mice without affecting airway eosinophilia while anti-IL-5 antibody did not reduce the airway hyperresponsiveness in one study (14), but deleting the IL-5 gene in C57BL/6 mice was found to abolish their ability to generate airway hyperresponsiveness and tissue eosinophilia in another (15). Now, three distinct pathways leading to airway hyperresponsiveness after T cell activation have been suggested (3). First, the IL-4 dependent pathway, which involves that IgE-stimulated activation of mast cells discharge mediators, such as histamine and leukotrienes that induce airway hyperresponsiveness upon exposure to the allergen (16). Second, the IL-5 dependent pathway, which involves allergen-activated T cell release of IL-5 that promotes eosinophil mediated epithelial cell damage and airway hyperresponsiveness (16). Finally, the IL-4 and IL-5 independent pathway, which involves a novel CD4+ T cell pathway for modulating airway hyperresponsiveness, was suggested to function in IL-4- and IL-5- deficient BALB/c mice (17). IL-13 shares a common alpha chain with IL-4 and enhances IgE production, mucus production, and eotaxin expression (3, 18). It has been reported that IL-13 can induce airway hyperresponsiveness independent of serum allergen-specific IgE, airway eosinophilia, IL-4, and IL-5; blockade of IL-13 before the allergen provocation resulted in decreased airway hypersensitivity without affecting airway eosinophilic inflammation or serum ovalbumin specific IgE level (18). IL-13 is important during antigen challenge, whereas IL-4 is useful during sensitization (18). This finding is concordant with our results. In the present study, no difference was found in the extents of the mainly IL-5 dependent processes (i.e., the proportion of eosinophils in BAL fluid, the degree of airway inflammation, or epithelial damage) or in the extent of the mainly IL-4 dependent process (i.e., serum allergen-specific IgE level) in mice that were rechallenged at different times. However, airway hyperresponsiveness persisted for only 7 weeks after the initial challenge. Therefore, an IL-4- and IL-5-independent regulatory mechanism, such as the regulation of IL-13 or novel CD4+ T cell pathway, might be involved in the modulation of airway hyperresponsiveness upon secondary allergen challenge. However, the actual mechanism of this phenomenon remains to be elucidated.
We should admit that the number of mice in each group might be small as this study was done as a preliminary experiment before the evaluation of a new therapeutic modality. There were some variation in the level of serum specific IgE and the average value might seem to be somewhat decreased in the last part of study. However, the levels of serum specific IgE in the challenged or rechallenged mice were not statistically different by Kruskall-Willis test (p>0.05) and definitely higher than that of PBS treated mice (p<0.05).
In summary, we report that airway inflammation and allergen specific IgE production may persist longer than airway hyperresponsiveness in a mouse asthma model of secondary allergen challenge. It is important to know the persistence of a disease phenotype in an animal model, especially in the evaluation of newly developed treatment modalities. This study also supports the hypothesis that airway inflammation, allergen-specific IgE production and airway hyperresponsiveness may be regulated by different mechanisms.
Figures and Tables
Fig. 1
Experimental protocols for sensitization, initial challenge and secondary challenge. 7-week old female BALB/c mice were used for the experiment. Mice were sensitized by intraperitoneal injection and initial provocative challenge was done on days 21, 22, 23 (0 week). Secondary challenges were performed at 5, 7, 9, or 12 weeks after the initial challenge. PBS treated mice were used as controls. Five mice were used in each group. IP: intraperitoneal injection.

Fig. 2
Airway hyperresponsiveness upon a secondary OVA challenge is persistent when mice were rechallenged 5 or 7 weeks after the initial inhalation challenge. However, airway hyperresponsiveness disappeared when mice were rechallenged 9 or 12 weeks after the initial challenge. The results are presented as PC200 values, where PC200 is the concentration of methacholine required to increase the baseline Penh by 200%.

Fig. 3
(A) The proportion of eosinophils in bronchoalveolar lavage fluid. Eosinophils are increased even when mice were rechallenged 12 weeks after the initial challenge. (*p<0.05, compared to the PBS treated mice, BALF: bronchoalveolar lavage fluid). (B) The degrees of airway inflammation observed in the initially and rechallenged animals at 5, 7, 9, or 12 weeks after the initial challenge are similar each other and higher than that of the PBS treated mice. Total lung inflammation was defined as the average value of the peribronchial and perivascular inflammation scores. (*p<0.05, compared to initially challenged mice).
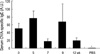
Fig. 4
The serum ovalbumin specific IgE level is still increased when mice were rechallenged 12 weeks after the initial challenge. The level of the specific IgE in the initially challenged or rechallenged mice are similar (p>0.05, by Kruskal-Willis test) and higher than that of PBS treated group (*p<0.05, compared to all allergen challenged groups). Sera of four to five mice were evaluated in each group. BALF: bronchoalveolar lavage fluid.
References
1. Kim YY, Cho SH, Kim WK, Park JK, Song SH, Kim YK, Jee YK, Ha MN, Ahn YO, Lee SI, Min KU. Prevalence of childhood asthma based on questionnaires and methacholine bronchial provocation test in Korea. Clin Exp Allergy. 1997; 27:761–768.

2. Beasly R. The burden of asthma with specific reference to the United States. J Allergy Clin Immunol. 2002; 109:Suppl 5. S482–S489.
3. Leong KP, Huston DP. Understanding the pathogenesis of allergic asthma using mouse models. Ann Allergy Asthma Immunol. 2001; 87:96–109.

4. Institute of laboratory animal resources, commission on life sciences, National research council, USA. Guide for the care and use of laboratory animals. 1996. Washington D.C: National academy press.
5. Park YJ, Chang YS, Lee SW, Cho SY, Kim YK, Min KU, Kim YY, Cho SH, Sung YC. The enhanced effect of a hexameric deoxyriboguanosine run conjugation to CpG oligodeoxynucleotides on the protection against allergic asthma. J Allergy Clin Immunol. 2001; 108:570–576.
6. Hamelmann E, Schwarze J, Takeda SK, Oshiba A, Larsen GL, Irvin CG, Gelfand E. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997; 156:766–775.

7. Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000; 30:79–85.

8. Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Thorne PS, Krieg AM. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998; 160:2555–2559.
9. Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998; 161:7054–7062.
10. Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J Immunol. 2000; 164:5575–5582.

11. Sur S, Wild JS, Choudhury BK, Sur N, Alam R, Klinman DM. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999; 162:6284–6293.
12. Kanehiro A, Ikemura T, Makela MJ, Lahn M, Joetham A, Dakhama A, Gelfand EW. Inhibition of phosphodiesterase 4 attenuates airway hyperresponsiveness and airway inflammation in a model of secondary allergen challenge. Am J Respir Crit Care Med. 2001; 163:173–184.

13. O'Byrne PM. Airway hyperresponsiveness. In : Middletone E., editor. Allergy principles & practice. 1998. 5th ed. St. Louis: Mosby;p. 859–866.
14. Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not Interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996; 183:109–117.

15. Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airway hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996; 183:195–201.
17. Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998; 161:1501–1509.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download