Abstract
We investigated the interrelations between surface electrocardiographic changes and clinical outcomes in children with idiopathic dilated cardiomyopathy (DCMP). 33 patients (19 boys, 14 girls) were classified into two groups; group I (15) who were in poor clinical status or dead; and group II (18) who showed good clinical status. Group I had larger LV dimensions compared to group II (Gr I vs. Gr II; LVEDD, 52±11 vs. 42±7 (mm); LVESD, 43±12 vs. 30±5 (mm); p<0.05). QRS duration was prolonged in Gr I compared to Gr II and normal (Gr I, 84±28; Gr II, 66±12; normal control, 67±9). The QRS duration was correlated with the dimensions of left ventricle (LV). Corrected QT and JT interval and dispersions of QT in the DCMP group showed a significant difference compared to the normal control, however there was no significant difference between Gr I and II. In conclusion, QRS duration was correlated with ventricular dimension and clinical outcome in children with idiopathic dilated cardiomyopathy. Irrespective of increased ventricular inhomogeneity, QT dispersion could not be used to predict long-term prognosis.
Dilated cardiomyopathy is a myocardial disease characterized by impaired systolic function and dilatation of the left or both ventricles. Total mortality is high, and approximately half of the deaths are sudden and unexpected. Ventricular arrhythmias, commonly observed in patients with heart failure, are thought to underlie at least some of sudden deaths (1, 2). With the increasing severity of heart failure, the number and complexity of ventricular arrhythmias also increase (3).
The published data on dilated cardiomyopathy in children are sparse and little is known about the long-term clinical course and the factors that may influence prognosis for better or worse (4-7). Children with idiopathic dilated cardiomyopathy also have arrhythmias; however, it has not been demonstrated whether the control of these arrhythmias alters clinical outcome.
The mechanism of arrhythmias that occur in the setting of heart failure is still unclear. Experimental evidence points to a higher tendency for the failing myocardium to develop early and delayed afterdepolarization-induced triggered activity and automaticity (8, 9). Conditions favoring reentry also have been described in failing hearts (10). Modulation factors such as sympathetic activation, electrolyte disturbances and chronic stretch are present in a heart failure setting and may favor all of the mentioned mechanisms of arrhythmias (11). The pathologic substrate of cardiomyopathy may create the conditions for nonhomogenous recovery of excitability. So far, only a limited amount of data has been published about QT dispersion in children with dilated cardiomyopathy.
This study aimed to investigate interrelations between surface electrocardiographic changes reflecting ventricular depolarization and repolarization inhomogeneity and clinical outcomes in children with idiopathic dilated cardiomyopathy.
Clinical records of patients with idiopathic dilated congestive cardiomyopathy were reviewed. Patients with any hemodynamically significant congenital heart defect, previous history of therapy with any antineoplastic agent or an inborn error of metabolism with potential for myocardial involvement were excluded from the study. None of the patients were considered to have cardiomyopathy as a result of primary arrhythmia.
Dilated cardiomyopathy was diagnosed on the basis of several sources such as history, physical examination, and two-dimensional echocardiography demonstrating a non-hypertrophied, dilated left ventricle. The absence of congenital or acquired cardiac disease was also assessed on the basis of history, physical examination, chest radiography and 2-dimensional echocardiography. None had undergone surgery that included a ventriculotomy. There were no known electrolyte abnormalities in the children, and none were taking any antiarrhythmic agents known to affect the QT interval.
Thirty-three patients were identified and their data were analyzed. There were 19 boys and 14 girls. The mean age at this study was 80±50 months (median 68, range 8-247 months). The mean age at diagnosis was 25±38 months. The mean duration of illness from diagnosis was 72±41 months (median 63, range 0-179). According to the clinical status at the last follow-up, they were classified into two groups; group I (15 patients; male 9, female 6) included patients with a poor ventricular function (NYHA class III or IV) or death; group II (18 patients; male 10, female 8) had those patients who showed improvement in ventricular function compared to their initial status (NYHA class I or II). There was no significant difference in the age distribution between group I and group II.
As controls, we selected a group of 20 normal subjects who were without any structural cardiac abnormality. Most of these controls were seen as outpatients referred for systemic checkups or were hospitalized patients undergoing prospective work-up before orthopedic or plastic surgery. The mean age was 89±41 months (median 79 months).
We analyzed the surface electrocardiograms (ECGs) of 33 children with idiopathic dilated cardiomyopathy and 20 normal age-matched children. Echocardiographic data were reviewed. Left ventricular and right ventricular enlargement was assessed by two-dimensional measurements. The surface ECG and two-dimensional echocardiographic data were obtained at the last follow-up.
The ECG recordings were taken with a paper speed of 50 mm/sec at normal filtering. Several ECG parameters were measured manually. QRS duration was defined as the maximum QRS duration in any lead from the first to the final sharp vector crossing the isoelectric line. QT interval was measured from the lead II using calipers. QT interval was defined as the interval between the beginning of QRS complex and the end of T wave. The onset and offset of T wave were defined as the intersections of the isoelectric line and the tangent of the maximal slope on the up and down limbs of T wave, respectively. Care was taken to avoid U waves in any measurement, and when U waves were present, the end of T wave was taken as the nadir between T and U waves. Three consecutive cycles were measured in each of the standard 12 leads, and a mean value was calculated from the three values. The JT interval was then calculated by subtracting QRS from QT in individual leads. Bazett's formula was used to obtain corrected QT and JT intervals and these were represented as QTc and JTc. The dispersion of QT intervals was defined as the difference between the maximum and minimum of QT interval which could be measured in any of the 12 ECG leads and was represented as QTd. At the time of QT evaluation, all patients were hemodynamically stable and none had electrolytic disturbances, atrial fibrillation or significant intraventricular conduction defects. No additional drugs other than digoxin, angiotensin converting enzyme inhibitors, or diuretics, were administered.
Statistical analysis was performed by using Kruskal-Wallis analysis. A p value of <0.05 was considered to be significant.
Five of the 33 patients died. The cause of death was mainly attributed to pump failure of the left ventricle. One patient died due to sustained ventricular tachycardia after Batista surgery.
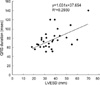
The dimensions of left ventricle (LVEDD, left ventricle end diastolic dimension; LVESD, left ventricle end systolic dimension) were correlated with QRS interval (LVEDD, y=1.0317x+26.713, R2=0.2491; LVESD, y=1.031x+37.654, R2=0.2939) (Fig. 1, 2). Group I had larger LV dimensions compared to those of group II (Gr I vs. Gr II; LVEDD, 52±11 vs. 42±7 mm; LVESD, 43±12 vs. 30±5 mm; p<0.05 respectively).
QRS duration was significantly prolonged in group I compared to both group II and the normal control (Gr I, 84± 28 msec; Gr II, 66±12 msec; normal control, 67±9 msec). QT and JT interval showed no significant difference among the three groups. But corrected QT and JT by Bazett's formula (QTc and JTc) were significantly different between the dilated cardiomyopathy groups (Gr I and II) and normal control. Dispersions of QT in the dilated cardiomyopathy groups (Gr I and II) were significantly prolonged compared to those of normal control. However, there were no significant differences between group I and II (Table 1).
Ventricular arrhythmias are reportedly common in children with dilated cardiomyopathy, and it might be a cause of sudden death (4, 5). In Greenwood et al.'s study, the presence or type of arrhythmia did not seem to alter the clinical outcome (4). However, Friedman et al. (7) reported that a high frequency of supraventricular arrhythmias was observed in 63 children with idiopathic dilated cardiomyopathy and the 5-yr survival rate was in the range of 80% with most deaths occurring in the first few years after diagnosis. Three of those 10 deaths were classified as sudden. It is very likely that the occurrence of sudden death is related to a rhythm disturbance. Patients who presented with ST or T wave changes or both on their ECG at baseline and at follow-up, also demonstrated a higher mortality rate.
A combination of increasing PR interval and QRS duration, particularly along with a rightward shift of QRS axis, appears to be a marker of high risk in patients with dilated cardiomyopathy (12). In our study, left ventricular dimensions were correlated to the clinical status. In patients who were in New York Heart Functional Class III or IV, the dimensions of the left ventricle were larger than those of patient with NYHA class I or II. QRS duration was correlated to the left ventricular dimensions. This indicates that QRS duration may reflect the dimension and muscle mass of the left ventricle and may be helpful to predict the clinical outcome. Regular check up of QRS duration on surface ECG might be necessary to predict the clinical outcome.
It is difficult to predict sudden and unexpected death in patients with dilated cardiomyopathy. Thus, it is very important to identify a good marker of patient susceptibility to the development of life-threatening ventricular arrhythmias. It is difficult to determine the exact underlying mechanism of ventricular arrhythmias in heart failure. Several studies have shown that hypertrophied and failing myocardium shows a higher propensity to develop early afterdepolarizations (8-10). Decreases in myocardial norepinephrine content and beta-receptor density were inhomogeneous over the failing heart (13, 14). Serum concentrations of potassium, magnesium and sodium are lower in patients with heart failure (15). Both increased catecholamine levels and electrolyte disturbances may favor the occurrence of delayed afterdepolarizations, triggered activity, and automaticity and thus, contribute to arrhythmogenesis in heart failure (16). Stretch is able to generate ventricular arrhythmias by all of the above mentioned arrhythmogenic mechanisms. Increased preload and/or afterload shorten repolarization in a failing ventricle and increases the inducibility of ventricular arrhythmias (17).
The dispersion of ventricular repolarization is thought to play an important role in the genesis of ventricular arrhythmias, and has been shown to reflect non-homogenous recovery of excitability within the myocardium. This provides a potential electrophysiological substrate leading to the occurrence of serious ventricular arrhythmias in various cardiac diseases (18). Increased dispersion in refractory periods has also been described in patients with left ventricular hypertrophy and can favor the occurrence of reentrant arrhythmias (19).
The relationship between the QTc interval and cardiomyopathy has not been well studied. An increased prevalence of prolonged QTc interval was seen in patients with dilated and hypertrophic cardiomyopathy (20). Barr et al. (21) showed that QT dispersion on a 12 lead ECG was a useful predictor of sudden death in a population suffering from chronic heart failure. Pye et al. (17) showed significantly greater mean QT dispersion in dilated cardiomyopathy patients with ventricular tachycardia (76±18 msec) than in controls (40±11 msec). Pinsky et al. (22) concluded that QT dispersion provides a powerful means to stratify a patient's risk of dying while awaiting heart transplantation. In contrast, Davey et al. (23) found that there was a non-significant tendency to increase QT dispersion in chronic heart failure and they found no correlation between increased QT dispersion and arrhythmias as measured on 24 hr Holter monitoring. Fei et al. (24) studied 135 consecutive patients with idiopathic dilated cardiomyopathy and also found no significant difference in QT dispersion between survivors and those who died or received transplantation. Zaidi et al. (25) reported that dispersion of ventricular repolarization is increased in patients with dilated cardiomyopathy, especially in those with ventricular conduction defects.
In this study, dispersion of QT in dilated cardiomyopathy was significantly prolonged than that of normal control. This indicates that ventricular inhomogeneity may increase in patients with dilated cardiomyopathy. However, QT dispersion did not show significant difference according to the clinical status and ventricular function. Consequently, QT dispersion could not be used in predicting the clinical outcome in children with dilated cardiomyopathy.
This study has all the limitations of having been retrospective. Patients were not followed up for a uniform time, nor at regular intervals. Patients were highly selected in having been referred and followed up at a tertiary center.
In conclusion, QRS duration was correlated with ventricular dimensions and clinical outcome. But irrespective of increased ventricular inhomogeneity, QT dispersion could not be used to predict long-term prognosis.
Figures and Tables
References
1. De Maria R, Gavazzi A, Caroli A, Ometto R, Biagini A, Camerini F. Ventricular arrhythmias in dilated cardiomyopathy as an independent prognostic hallmark. Italian Multicenter Cardiomyopathy Study (SPIC) Group. Am J Cardiol. 1992. 69:1451–1457.
2. Bjarnason I, Hardarson T, Jonsson S. Cardiac arrhythmias in hypertrophic cardiomyopathy. Br Heart J. 1982. 48:198–203.

4. Greenwood RD, Nadas AS, Fyler DC. The clinical course of primary myocardial disease in infants and children. Am Heart J. 1976. 92:549–560.

5. Taliercio CP, Seward JB, Driscoll DJ, Fisher LD, Gersh BJ, Tajik AJ. Idiopathic dilated cardiomyopathy in the young: clinical profile and natural history. J Am Coll Cardiol. 1985. 6:1126–1131.

6. Griffin ML, Hernandez A, Martin TC, Goldring D, Bolman RM, Spray TL, Strauss AW. Dilated cardiomyopathy in infants and children. J Am Coll Cardiol. 1988. 11:139–144.

7. Friedman RA, Moak JP, Garson A Jr. Clinical course of idiopathic dilated cardiomyopathy in children. J Am Coll Cardiol. 1991. 18:152–156.

8. Vermeulen JT. Mechanisms of arrhythmias in heart failure. J Cardiovasc Electrophysiol. 1998. 9:208–221.

9. Xiao HB, Roy C, Fujimoto S, Gibson DG. Natural history of abnormal conduction and its relation to prognosis in patients with dilated cardiomyopathy. Int J Cardiol. 1996. 53:163–170.

10. de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, Dingemans KP, van Hemel NM, Hauer RN. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988. 77:589–606.

11. Aronson RS. Afterpotentials and triggered activity in hypertrophied myocardium from rats with renal hypertension. Circ Res. 1981. 48:720–727.

12. Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992. 85:1046–1055.

13. Pierpont GL, Francis GS, DeMaster EG, Olivari MT, Ring WS, Goldenberg IF, Reynolds S, Cohn JN. Heterogeneous myocardial catecholamine concentrations in patients with congestive heart failure. Am J Cardiol. 1987. 60:316–321.

14. Beau SL, Tolley TK, Saffitz JE. Heterogeneous transmural distribution of beta-adrenergic receptor subtypes in failing human hearts. Circulation. 1993. 88:2501–2509.

15. Dargie HJ, Cleland JG, Leckie BJ, Inglis CG, East BW, Ford I. Relation of arrhythmias and electrolyte abnormalities to survival in patients with severe chronic heart failure. Circulation. 1987. 75(5 Pt 2):IV98–IV107.
16. Coronel R, Fiolet JW, Wilms-Schopman FJ, Schaapherder AF, Johnson TA, Gettes LS, Janse MJ. Distribution of extracellular potassium and its relation to electrophysiologic changes during acute myocardial ischemia in the isolated perfused porcine heart. Circulation. 1988. 77:1125–1138.

17. Pye MP, Cobbe SM. Arrhythmogenesis in experimental models of heart failure: The role of increased load. Cardiovasc Res. 1996. 32:248–257.

18. Vassallo JA, Cassidy DM, Kindwall KE, Marchlinski FE, Josephson ME. Nonuniform recovery of excitability of the left ventricle. Circulation. 1988. 78:1365–1372.
19. Zaidi M, Robert A, Fesler R, Derwael C, Brohet C. Dispersion of ventricular repolarization in hypertrophic cardiomyopathy. J Electrocardiol. 1996. 29:Suppl. 89–94.

20. Martin AB, Garson A Jr, Perry JC. Prolonged QT interval in hypertrophic and dilated cardiomyopathy in children. Am Heart J. 1994. 127:64–70.

21. Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994. 343:327–329.

22. Pinsky DJ, Sciacca RR, Steinberg JS. QT dispersion as a marker of risk in patients awaiting heart transplantation. J Am Coll Cardiol. 1997. 29:1576–1584.

23. Davey PP, Bateman J, Mulligan IP, Forfar C, Barlow C, Hart G. QT interval dispersion in chronic heart failure and left ventricular hypertrophy: relation to autonomic nervous system and Holter tape abnormalities. Br Heart J. 1994. 71:268–273.

24. Fei L, Goldman JH, Prasad K, Keeling PJ, Reardon K, Camm AJ, McKenna WJ. QT dispersion and RR variations on 12 lead ECGs in patients with congestive heart failure secondary to idiopathic dilated cardiomyopathy. Eur Heart J. 1996. 17:258–263.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download