Abstract
To assess the effectiveness of endovascular stenting for the palliation of superior vena cava (SVC) syndrome, endovascular stent insertion was attempted in 10 patients with symptomatic occlusion of the SVC. All the patients had known malignant disease of the thorax. Eight patients had been treated previously with chemotherapy and radiotherapy (n=5), chemotherapy alone (n=2), or pneumonectomy and radiotherapy (n=1). After developing SVC syndrome, all the patients were stented before receiving any other treatment. After single or multiple endovascular stents were inserted, five of eight patients were treated with chemotherapy and radiotherapy (n=2) or chemotherapy alone (n=3). Resolution of symptoms was achieved in nine patients within 72 hr (90%). In one patient, the symptoms did not disappear until a second intervention. At follow up, symptoms had recurred in two of ten patients (20%) after intervals of 15 and 60 days. Five patients have died from their cancers, although they remained free of symptoms of SVC occlusion until death. In conclusion, endovascular stent insertion is an effective treatment for palliation of SVC syndrome. Endovascular stent insertion can be considered the first choice of treatment, due to the immediate relief of symptoms and excellent sustained symptomatic relief.
Obstruction of the superior vena cava (SVC) is a recognized complication of lung cancer. Before the mid twentieth century, malignancy accounted for one-third of all cases of SVC syndrome (SVCS). Most cases occurred secondary to benign disease (1). Today, however, intrathoracic malignancy has far surpassed benign disease as the primary cause of SVC obstruction. Approximately 73 to 97% of SVCS cases occur secondary to malignancy, and the most frequent cause is lung malignancy. Approximately 3 to 5% of patients with lung malignancy develop the syndrome (2). Obstruction of the SVC occurs either via direct extension or compression due to the primary tumor or via invasion of the mediastinal lymph nodes. In addition, progressive tumor growth may violate the vascular intima and serve as a nidus for thrombus formation, which can evolve to extensive thrombosis of vessels.
SVC syndrome due to malignancy produces acute distress and degrades the quality of life during the limited survival. Therefore, the goal of SVCS therapy is rapid and effective palliation of the symptoms, rather than long-term remission. Traditionally, most patients with SVCS secondary to malignancy have been treated non-operatively, with radiotherapy, chemotherapy, or both. With radiotherapy, diminished venous distension and subjective improvement usually do not occur until three to seven days after beginning therapy. Approximately 46 to 70% of patients with bronchogenic carcinoma will demonstrate a symptomatic response to radiotherapy or combined radiotherapy and chemotherapy within the first two weeks (3, 4).
More recently, endovascular stents have been used successfully to alleviate symptoms. Prompt, persistent resolution of symptoms is achieved in 75 to 95% of patients (5, 6). This paper reviews our experience in treating SVCS to assess the effectiveness of endovascular stenting for palliation of SVCS.
Between September 2001 and February 2003, percutaneous endovascular stent (Wall Stent, Boston Scientific, Nastick, MA, U.S.A.) insertion was attempted in 10 patients (8 men, 2 women), age range 37-63 (mean 54) yr, with symptomatic occlusion of the SVC. Wall stents varied in length (4-10 cm) and diameter (10-14 mm). The most commonly used was 8 cm×14 mm. All patients had known malignant disease of thorax (squamous cell carcinoma: 4, adenocarcinoma: 3, poorly differentiated carcinoma: 3). Eight patients had been treated previously with chemotherapy and radiotherapy (n=5), chemotherapy alone (n=2), or pneumonectomy and radiotherapy (n=1).
After developing SVCS, all the patients were stented before attempting any other palliative treatment. Digital subtraction angiography was performed before stenting to localize the site of obstruction. The SVC was stenosed or occluded in all cases by tumor and thrombotic occlusion of the SVC. Venous access was achieved via the right jugular vein in four patients, via the left jugular vein in one patient, and via the right femoral vein in five patients. After navigating the stenosed or occluded segment of the SVC with a angiographic catheter and guidewire, one (n=7) or two (n=3) endovascular stents were inserted. Catheter-directed thrombolysis was not used. Primary clinical patency was defined as the resolution of edema after the procedure; if another procedure was needed to relieve SVCS, it was recorded as secondary clinical patency.
After stenting, five patients were treated with either chemoradiation (n=2) or chemotherapy alone (n=3). Retrospective analysis of the clinical records was used to assess the effectiveness of endovascular stenting for the palliation of SVCS.
Stent placement was successful in all patients. Symptom resolution was achieved in nine patients within 72 hr (90%). Initial clinical success was achieved in 90% (9/10). Primary clinical patency was achieved in 77.8% (7/9). At follow up, symptoms due to thrombosis recurred in two of nine patients after 15 or 60 days; both were successfully resolved by repeated stenting (Fig. 1). Therefore, secondary clinical patency rate was 100%. These two patients took part early in the trial, when we did not use anticoagulation or antiplatelet therapy. Subsequently, warfarin 5 mg and acetyl salicylic acid 300 mg were used to prevent thrombosis.
Symptom-free survival ranged from 12 days to 14 months (mean 6.7 months). Presently, five patients are alive, remain asymptomatic, and have a patent stent. Five patients have died from their cancers; however, they remained free of symptoms of SVC occlusion until death.
Superior vena cava syndrome generally occurs as a result of either compression by an adjacent tumor or compression by lymph nodes. A tumor invades the vena cava much less frequently in advanced stages of disease (7). Patients with SVCS from lung malignancy have a very poor prognosis. The response to therapy in patients with lung malignancy and SVCS may have an impact on survival. In one series, those who did not respond to non-operative therapy within 30 days had a significantly lower one-year survival (7%) than those who responded to therapy (17 to 24%). Patients who received no therapy or who developed changes in mentation and compromised airways had a median survival of only six weeks (3, 8, 9).
Four types of treatment are available for patients with SVCS. General medical treatment (i.e., bed rest, elevation of the head, diuretics, steroids, and anticoagulation) is of limited clinical benefit (2). Radiation therapy is widely advocated for SVCS caused by radiosensitive tumors and provides relief by reducing tumor volume (10). Radiotherapy and chemotherapy are the standard forms of treatment, and a combination of the two has been used (11). Symptom improvement of 75-90% has been reported, although other studies have obtained less promising results (46% success with radiation therapy for non-small cell carcinoma and 62-80% with chemotherapy for small cell carcinoma) (11, 12).
Traditional therapies require 2-4 weeks to show an effect (11, 13). Furthermore, SVCS recurs in 20-50% of cases and only symptomatic treatment is possible. More aggressive radiation therapy has achieved responses of up to 90%. Nevertheless, high-dose radiation therapy has many complications. Since these affect the quality of life of a patient whose life expectancy is only 6 months, they may be considered unacceptable and counterproductive, as radiation therapy can induce fibrotic changes that further constrict the vessels.
After Charnsangavej et al. (14) introduced endovascular stenting in dogs to treat obstruction of the vena cava, the development of endovascular stenting has continued. The procedure is easy to perform and well tolerated by patients. Complications are minimal and, in terms of symptom relief, the results are encouraging. The Wall stent is the most common endovascular device used to treat SVCS, although the Palmaz and Gianturco stents have also been used with success.
We attempted percutaneous endovascular stent (Wall Stent, Boston Scientific, Nastick, MA, U.S.A.) insertion in 10 patients. The efficacy of stenting a central venous obstruction was clear. Nicholson et al. (13) presented convincing data comparing palliative radiation therapy and endovascular stenting. Symptom improvement usually occurred within 48-72 hr of stent placement and, 90% of patients remained symptom-free until death, as compared with only 12% of those who were treated with palliative radiation therapy. They concluded that stent placement was a more effective palliative therapy and recommended stenting as the procedure of choice.
In the Cochrane systematic review (15) of treatment for malignant SVC obstruction, stent insertion provided relief of symptoms more rapidly, and in a higher proportion of patients (95%) than did radiation therapy or chemotherapy. Others have reported success rates of 90 to 100% in patients with malignancy-related SVCS treated with combined endovascular therapy (thrombolysis, angioplasty, and stent placement) (16). Kee et al. (6) reported technical success in 56 of 59 patients (95%), primary clinical patency was 79%, and secondary clinical patency was 93% with mortality and morbidity rates of 3 and 10%, respectively. In our patients, initial clinical success was achieved in 90% (9/10). Primary clinical patency was achieved in 77.8% (7/9). At follow up, symptoms due to thrombosis recurred in two of nine patients after 15 or 60 days; both were successfully resolved by repeated stenting. In our patients, secondary clinical patency was 100%.
Gross et al. (17) reported no recurrences in 13 patients, with follow-ups ranging from 3 to 180 days. Rosch et al. (18) had 1 recurrence in a group of 20 patients, with follow-ups ranging from 1 to 11 months. The reported recurrence rate ranges from 0 to 45%. In our patients, symptoms recurred in two of nine patients (22.2%) after 15 and 60 days, both were thrombotic occlusions and were relieved by placement of another stent.
The most common complication of this therapy is stent thrombosis, which has been successfully treated with thrombolysis or further stent insertion (19). Early (within one month of stenting) thrombosis occurs in 8-20% and late (more than one month after endovascular stenting) thrombosis occurs in 5-45%. Thrombosis is significantly reduced when long-term anticoagulation (warfarin, platelet inhibitors) is used following endovascular stenting (17). After we experienced one early and another late thrombosis, we began to use warfarin and platelet inhibitors. Since no thrombosis occurred during the long-term follow-up period, this treatment seems adequate to prevent further thrombosis.
In summary, our results with endovascular stents in the initial treatment of SVCS of malignant cause were excellent. Percutaneous endovascular stent insertion is an effective treatment for palliation of SVC syndrome. Endovascular stent insertion is the first choice of treatment as it produces immediate relief of symptoms and excellent sustained symptomatic relief. Furthermore, stent insertion does not interfere with subsequent radiation or chemotherapy. The immediate and late complications that can arise are preventable and easily resolved.
Figures and Tables
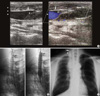
Fig. 1
(A) Venous Doppler of superior vena cava demonstrating near total obstruction (arrowheads) in the first stent (arrows), 2 weeks after the first procedure. (B) Fluoroscopic images show sequence of procedure for placement and balloon dilatation of 6 cm×14 mm the second stent through the first stent, across the lesion. (C) Radiography showing two stents placement, after the second procedure. Arrow and arrowhead indicate the upper ends of the second and first stent respectively.
References
1. Gomes MN, Hufnagel CA. Superior vena cava obstruction: a review of the literature and report of 2 cases due to benign intrathoracic tumors. Ann Thorac Surg. 1975. 20:344–359.
2. Escalante CP. Causes and management of superior vena cava syndrome. Oncology. 1993. 7:61–68.
3. Armstrong BA, Perez CA, Simpson JR, Hederman MA. Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys. 1987. 13:531–539.

4. Davenport D, Ferree C, Blake D, Raben M. Response of superior vena cava syndrome to radiation therapy. Cancer. 1976. 38:1577–1580.

5. Eng J, Sabanathan S. Management of superior vena cava obstruction with self-expanding intraluminal stents. Two case reports. Scand J Thorac Cardiovasc Surg. 1993. 27:53–55.

6. Kee ST, Kinoshita L, Razavi MK, Nyman UR, Semba CP, Dake MD. Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology. 1998. 206:187–193.

7. Qanadli SD, El Hajjam M, Mignon F, de Kerviler E, Rocha P, Barre O, Chagnon S, Lacombe P. Subacute and chronic benign superior vena cava obstructions: endovascular treatment with self-expanding metallic stents. Am J Roentgenol. 1999. 173:159–164.

8. Stanford W, Doty DB. The role of venography and surgery in the management of patients with superior vena cava obstruction. Ann Thorac Surg. 1986. 41:158–163.

9. Maddox AM, Valdivieso M, Lukeman J, Smith TL, Barkley HE, Samuels ML, Bodey GP. Superior vena cava obstruction in small cell bronchogenic carcinoma. Clinical parameters and survival. Cancer. 1983. 52:2165–2172.

10. Levitt SH, Jones TK Jr, Kilpatrick SJ Jr, Bogardus CR Jr. Treatment of malignant superior vena cava obstruction: a randomized study. Cancer. 1969. 24:447–451.
11. Urban T, Lebeau B, Chastang C, Leclerc P, Botto MJ, Sauvaget J. Superior vena cava syndrome in small-cell lung cancer. Arch Intern Med. 1993. 153:384–387.

12. Wurschmidt F, Bunemann H, Heilmann HP. Small cell lung cancer with and without superior vena cava syndrome: a multi-variate analysis of prognostic factors in 408 cases. Int J Radiat Oncol Biol Phys. 1995. 33:77–82.
13. Nicholson AA, Ettles DF, Arnold A, Greenstone M, Dyet JF. Treatment of malignant vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol. 1997. 8:781–788.
14. Charnsangavej C, Carrasco CH, Wallace S, Wright KC, Ogawa K, Richli W, Giantureo C. Stenosis of the vena cava: preliminary assessment of treatment with expandable metallic stents. Radiology. 1986. 161:295–298.

15. Rowell NP, Gleeson FV. Steroids, Radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus (Cochrane review). Cochrane Database Syst Rev. 2001. 4:CD001316.
16. Schindler N, Vogelzang RL. Superior vena cava syndrome. Experience with endovacular stents and surgical therapy. Surg Clin North Am. 1999. 79:683–694.
17. Gross CM, Kramer J, Waigand J, Uhlich F, Schroder G, Thalhammer C, Dechend R, Gulba DC, Dietz R. Stent implantation in patients with superior vena cava syndrome. Am J Roentgenol. 1997. 169:429–432.

18. Rosch J, Uchida BT, Hall LD, Antonovic R, Petersen BD, Ivancev K, Barton RE, Keller FS. Gianturco-Rosch expandable Z-stents in the treatment of superior vena cava syndrome. Cardiovasc Intervent Radiol. 1992. 15:319–327.
19. Stock KW, Jacob AL, Proske M, Bolliger CT, Rochlitz C, Steinbrich W. Treatment of malignant obstruction of the superior vena cava with the self-expandig Wall stent. Thorax. 1995. 50:1151–1156.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download