Abstract
Sclerosing hemangiomas (SH) of the lung are uncommon tumors and are thought to be benign. However, the biologic behavior of this tumor has not yet been characterized adequately. The clinicopathologic features were reviewed and analyzed for 16 cases of SH. The age of the patients ranged from 37 to 73 yr (mean 50.6 yr). There were fifteen female and one male patient. The SH located at the intraparenchyme in 14 cases, the interlobar fissure in one case and the visceral pleura in one case. The size of SH ranged from 0.3 cm to 8 cm (mean 2.6 cm). There were five unusual presentations of SH including a case having two SH with multiple nodules of atypical adenomatous hyperplasia in the same lobe, a case showing adenocarcinoma-like area within the SH, a case showing one peribronchial lymph node metastasis (N1 nodal stage) with location of interlobar major fissure, a case showing alveolar adenoma-like area within the SH, and one case with a large visceral pleural-based pedunculated mass presenting as mediastinal mass. All patients were alive and well without recurrence at the last follow up. Here, we reviewed previously published literatures and discussed the histogenesis of SH.
Pulmonary sclerosing hemangioma (SH) was first described by Liebow and Hubbell in 1956 (1). SH is thought to be a rare benign neoplasm, but it has uncertain histogenesis and biologic behavior. SH comprises a mixture of four histologic patterns, such as papillary, sclerotic, solid and hemorrhagic. SH consists of two cell types; eosinophilic cuboidal epithelial lining cells and round cells with either eosinophilic or clear cytoplasm forming sheets. The lining epithelial cells are termed as surface or cuboidal cells, while the other as round, stromal, or lesional cells. Although SH originally termed a variant of hemangioma (1), subsequent authors have suggested the origin of SH as mesenchymal (2), mesothelial (3) and neuroendocrine (4) derivation. Now there is a consensus that they are epithelial tumors (5, 6). The finding that SH has features of a neoplasm was the reason for its being moved from the category of tumor-like lesions in the 1981 World Health Organization (WHO) classification of lung tumors (7) to the category of miscellaneous tumors in the new 1999 WHO/International Association for the Study of Lung Cancer classification (IASLC) (8).
We reviewed the clinicopathologic features of sixteen cases diagnosed as SH including five cases with unusual presentations.
All sixteen cases of pulmonary SH diagnosed in the Department of Pathology, Samsung Medical Center, Seoul, Korea, from 1996 to September, 2003 were retrieved (Table 1). The surgical procedures included 11 cases of lobectomy and 5 cases of wedge resection. All tumor tissues less than 5 cm were totally embedded in paraffin block. Hematoxylin and eosin-stained slides were reviewed to reconfirm the diagnosis and to find the multiple histologic features by two experienced pathologists. Medical records and surgical pathology reports were also reviewed. Immunohistochemical stainings were performed on paraffin-embedded tissue of all 16 cases using labeled streptavidin-biotin peroxidase technique. The panel of antibodies included anti-thyroid transcription factor-1 (TTF-1) (1:50, Dako, Glostrup, Denmark), anti-pancytokeratin (AE1/3, 1:80, Zymed, San Francisco, CA, U.S.A.), anti-epithelial membrane antigen (EMA) (1:50, Dako), antisynaptophysin (1:40, Dako), chromogranin (1:2,000, Dako), CD34 (1:100, Dako), CD56 (1:20, Monosan, Uden, Netherlands), smooth muscle actin (SMA) (1:100, Dako), p53 protein (BP 53.12, 1:400, Zymed) and Ki-67 (1:100, Dako). A case was regarded as positive for TTF-1 if more than 10% of the specific cell population showed nuclear staining.
The mean age of the patients ranged from 37 to 73 yr (mean 50.6 yr). Among the 16 cases, there was marked female predominance, with a female to male ratio of 15:1. One case of SH was located at the interlobar fissure of the lung, one was a visceral pleural-based pedunculated lesion and the rest were intraparenchymal lesion. Six tumors were located at the left lower lobe, four at the right middle lobe, three at right lower lobe, two at the left upper lobe, and one at the right upper lobe. The size of SH ranged from 0.3 cm to 8 cm (mean 2.6 cm) in 17 masses out of 16 cases. Macroscopically, the tumors were rather well circumscribed and mostly pale yellowish and dark red in color if they were of hemangiomatous variety. The SH showed four distinct histologic patterns: papillary (Fig. 1A), solid (Fig. 1B), sclerotic (Fig. 1C) and hemorrhagic (Fig. 1D). The major histologic patterns composed of a variable proportion of all four patterns in 9 out of 17 masses. Hemorrhagic pattern was observed in all except for the smallest one (0.3 cm). All tumors composed of more than two histologic patterns and none demonstrated a single pattern (Table 1). The tumors were composed of varying proportions of two cell types, namely surface 'lining cells' or 'cuboidal', and pale polygonal 'lesional cells' or 'round cells'. These lining cells resembling type II alveolar cells were most prominent in the areas with papillary pattern (Fig. 1A). The round lesional cells were pale round cells showed uniform, medium-size polygonal nuclei with moderate amounts of pale, eosinophilic or clear cytoplasm. The round cells were distributed in the interstitial portion (Fig. 1A, B). Four out of 16 cases showed vacuolated clear epithelial lining cells. These cells reacted with TTF-1, EMA and pancytokeratin immunostaining. Mitotic figures were rarely seen in the cell population of the two cases. There were scattered alveolar macrophages, multinucleated giant cells, clumps of hemosiderin pigments, mast cells and eosinophils. Occasionally psammoma bodies were seen in five cases, cholesterol clefts in three cases and intralesional adipose tissue in one case. Also seen were necroses in four cases. Lamellar bodies were present in four cases (Table 2). Immunohistochemical study showed that both surface cells and round cells of SH were diffuse positive for TTF-1 in nucleus (Fig. 1E) and EMA in cytoplasm (Fig. 1F), and negative for synaptophysin, chromogranin, CD34 and SMA in all cases. CD56 focally reacted with the round cells in two cases (Fig. 1G). Cytokeratin (AE1/3) reacted with the surface cells in all cases. However, round cells rarely reacted with cytokeratin except one case that showed focal positivity for cytokeratin (Fig. 1H). The index of positivity of p53 protein ranged from negative to 20% (mean 6%) and the Ki-67 labeled proliferating index ranged from negative to 10% (mean 4.5%).
We experienced five unusual presentations of SH including one case with two nodules (1.2 cm and 0.3 cm) and multiple small atypical alveolar hyperplasia (AAH)-like nodules in background of the lung parenchyma. One case showed papillary adenocarcinoma-like area, and one case with one SH having regional lymph node metastasis. One case showed an alveolar adenoma-like area, and one case revealed a visceral pleural-based pedunculated mass that presented as a mediastinal mass clinically. The first case is a 42-yr-old female patient who was found to have a right lower lobe mass on chest radiograph without subjective symptoms. The surgeon found two nodules of the same lobe in the operating field. The size of the two tumors were 1.2 cm and 0.3 cm, respectively (Fig. 2A, B). The first case of 1.2 cm sized nodule showed four histologic patterns of papillary, solid, sclerotic and hemorrhagic, and focal mild atypia. The second case of the smaller nodule showed two histologic patterns of papillary and sclerotic. We could also find twelve small-sized AAH-like nodules with some papillae and micropapillae in background lung parenchyma of same lobe (Fig. 2C). The histology was similar to that of alveolar adenoma or AAH, but these had papillary or micropapillary structure and variably distributed irregular pattern. The tumor cells showed interstitial oval to spindle cell proliferation. The size of the small nodules ranged from 0.1 cm to 0.7 cm (mean 0.35 cm). Immunohistochemically, the lining cells of the small nodular lesion reacted with TTF-1 and EMA, and the interstitial stromal cells were focally positive for TTF-1 (Fig. 2D) and EMA. The second was a 37-yr-old female patient who presented with a 2 cm sized SH with one peribronchial regional lymph node metastasis (nodal stage, N1). A metastatic focus of the lymph node, measuring 0.1 cm, was noted. The metastatic tumor cells showed predominantly papillary structure lined by cuboidal cells and round cells (Fig. 2E). Both tumor cells also reacted with TTF-1 and EMA immunostaining. The third case was a 36-yr-old female patient with a 3 cm sized tumor with alveolar adenoma-like area peripherally to the main mass. The alveolar adenoma-like area continued to the main mass and had thin-walled multiple cystic spaces lined by low cuboidal cells, measuring 1 cm in length (Fig. 2F). The fourth case was a 59-yr-old female patient who presented with a mediastinal mass. Chest computed tomography showed a large pleural based mass that was located adjacent to the right cardiac border. On gross examination, the mass measured 8 cm in the greatest diameter, and was in continuity with the right middle lobe of the lung. The tumor displayed three major histologic patterns of SH; predominantly hemorrhagic, solid and papillary. The areas showing hemorrhagic pattern were composed of large blood-filled spaces lined by epithelial cells and were reminiscent of a cavernous hemangioma (Fig. 1D). This case was proven as SH by gross examination, operative record, histologic features and immunohistochemical study. The last case was a 43-yr-old female patient with one 1.3 cm sized tumor with an adenocarcinoma-like area, measuring 0.4 cm in the longest diameter (Fig. 2G). But there were no mitotic figures and no definite stromal invasion. Flow cytometery for the SH was performed and showed diploid. The Ki-67 labeled proliferating index and the index of p53 protein were 5% and 20%, respectively. The histologic pattern was mixed with hemorrhagic, papillary and sclerotic.
All 16 patients were alive and well without recurrence or metastasis during a follow-up period of 1 month to 6.8 yr (mean 2.3 yr).
SH is a rare primary pulmonary tumor and is thought to be benign tumor. However, the biologic behavior of this tumor has not yet been characterized adequately. The histogenesis of the SH also remains a subject of debate. Although SH originally termed a variant of hemangioma (1), subsequent authors have suggested the origin of SH as mesenchymal (2), mesothelial (3) and neuroendocrine (4) derivation. Now, there is a consensus that they originated from primitive epithelial cells (5, 6). The epithelial origin of the tumor was also supported by TTF-1 and EMA positivity in both the surface cells and round cells in the majority of the cases (6, 9). TTF-1 is expressed in the thyroid, lung, and the diencephalons of the brain. In the lung, it binds to the surfactant A, B, C and Clara cell secretory protein genes. It is expressed in the non-ciliated columnar cells of the fetal lung as early as 11 weeks of gestation, and in type II pneumocytes and Clara cells of the adult lung (10, 11). The TTF-1 expression of both cells supported that both cells were derived from a primitive respiratory cell or may represent incompletely differentiated pneumocytes or Clara cells (5, 6, 12). Pancytokeratin (AE1/3) usually reacts with surface cells, and not with round cells. However, round cells rarely reacted with cytokeratin except one case that showed focal positivity for cytokeratin. The immunohistochemical findings of the round cells were positive for TTF-1 and cytokeratin, suggesting that intermediate differentiation between differentiated epithelial cuboidal cells and undifferentiated round pale cells (5, 6).
Although it is thought to be benign, rare cases apparently showed metastatic potential. In 1986, Tanaka and colleagues reported the first case of SH with lymph node metastasis (13). We found thirteen cases in nine previously reported literatures with metastasizing pulmonary SH (Table 3) (5, 6, 13-20). Including the current case, there were seven cases of single lymph node metastasis and six cases of multiple (>2) lymph nodes metastases. Two cases of mediastinal (nodal stage, N2) lymph node metastases were found (17, 18). Distant hematogenous metastasis from SH has not been reported. The primary tumor size ranged from 1.5 to 10 cm (mean 4.7 cm). We assumed that the primary tumor size and male ratio in the metastasizing pulmonary SH had increased as compared to the population with no lymph node metastasis, however we do not have statistical data. In the 9 cases, including one current case, their follow-up periods were available. The mean time was 3.4 yr (ranging from 1 to 10 yr) (Table 3). The metastatic lymph node of the current case also showed both surface and round cells like that of previously described cases. There was no recurrence or no metastasis during the follow-up period. The prognosis did not appear to be affected by the presence of regional lymph node metastases. There were evidences which regarded both surface and round cells as neoplasia. A study using microdissection technique demonstrated that both surface cells and round cells of SH showed similar clonality and represented a variable differentiation from a common progenitor cell (21).
A few series have described multiple SH (6, 14, 22-29) and the incidence was 4-5% (6, 24, 26, 28). To our knowledge, two cases of bilateral multiple SH were described in the English-language literature (27, 29). The possible pathogenesis of multiple SH has not been established. The cases of AAH in continuity with multiple SH (25), alveolar adenoma with SH (17), and transition from atypical hyperplasia of type II pneumocyte to SH (29) have been described. The reports suggested that AAH of type II pneumocytes could be a focus of atypical growth of lining epithelial cells of SH. In our series, there was one case of alveolar adenoma-like area in the peripheral portion of the SH. Another case with two SH had a background of multiple AAH with papillae in the right lower lobe. We did not investigate other studies for the postulation except for an immunohistochemical study. We could not verify whether this case indicated multicentric origin of the SH or intrapulmonary metastasis, or incidental findings. Even if the AAH-like lesion with papillae is presumptive precursor lesion of SH, there are limitations for the postulation so far. A longer follow-up data, ample cases, and further studies are needed to support that.
The SH could be presented clinically with mediastinal mass, and be located at the interlobar fissure (6, 30). These histologic patterns were predominantly hemorrhagic or angiomatous like that of cavernous hemangioma. Sakamoto et al. (31) reported a case of SH isolated in the mediastinum, and suggested the hypothesis of three mechanism that may account for the development of mediastinal SH. The first was metastasis from the lung to the mediastinum. The second mechanism was that the tumor could have originated from ectopic lung tissues, such as bronchogenic cysts. The final mechanism was that the SH developed from the lung as a pedunculated pleural mass that slowly pulled away from the lung surface. All three of these potential mechanisms are plausible; however, the first mechanism was against findings that all previously reported cases of lymph node metastasis from SH had evidence of primary tumor. A case report of SH arising in the extrapulmonary sequestration was described in the English literature and may support the second mechanism (32). In the cases that presented as a pleural based or mediastinal mass, the contributor did not initially consider the diagnosis of SH. However, after careful review of the operative findings, chest radiographs, and histologic findings, it was apparent that these lesions arose from the periphery of the lung and protruded from the visceral pleural surface, giving the initial impression of an extrapulmonary origin (6, 33). Our current case which presented mediastinal mass also revealed a pulmonary origin after check of additional gross examination, operative findings, and compatible immunohistochemical studies.
Based on the findings of higher incidence in females, Leong et al. (28) and Aihara and Nakajima (34) studied both estrogen receptors (ER) and progesterone receptors (PR) immunohistochemically. They reported that most patients were positive for both ER and PR, suggesting a relationship between this tumor and female sex hormones.
Variable degrees of cytologic atypia were found in most SH. In our series, one case showed a focus of papillary adenocarcinoma-like area within the SH, measuring 0.4 cm in the longest diameter, and mitotic figure was not absent. Fine needle aspiration was performed in ten out of sixteen patients of our series. The diagnoses included insufficient specimen in four, malignancy versus SH in two and suspicion of adenocarcinoma in four. The severe cytologic atypia made it difficult to make a diagnosis of SH in aspiration cytology. The above findings; lymph node metastasis, severe atypia, and multifocal distribution, may suggest that SH is a low grade malignancy. However, SH is a clinically benign tumor and has a good prognosis. All our current cases showed no recurrent or metastasis after excision, and the patients all are alive and well at present.
In conclusion, SH may show hilar or mediastinal lymph nodes metastases, cytologically severe atypia, and multicentricity. But, these unusual features do not affect the prognosis. Complete excision of lesion is a curable treatment method and leads to an excellent prognosis. Further studies on many cases of SH are necessary to elucidate the mechanism of metastasis, multiplicity, and extrapulmonary manifestation.
Figures and Tables
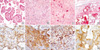 | Fig. 1Four major histologic patterns and immunohistochemistry of the sclerosing hemangiomas. Sclerosing hemangiomas show papillary (A), solid (B), sclerotic (C), and hemorrhagic (D) patterns. Sclerosing hemangioma consists of lining cuboidal cells (A) and stromal round cells (B) (H&E stain: A, B ×200; C, ×100; D, ×1). Immunohistochemical stain shows that both lining cells and round cells are positive for TTF-1 (E) and EMA (F), and CD56 (G). The pancytokeratin (H) reacts with the lining cells and focally reacts with round cells. (E to H, ×200). |
 | Fig. 2Unusual presentations of the sclerosing hemangiomas. Two sclerosing hemangiomas (A, B) with atypical alveolar hyperplasia-like nodule (C) in the background lung parenchyme (H&E stain: A, B, ×1; C, ×200). Atypical alveolar hyperplasia-like nodule shows that the lining cells and some stromal cells are positive for TTF-1 (D) immunostaining (×400). (E) One sclerosing hemangioma with lymph node metastasis (H&E stain, ×200). (F) One sclerosing hemangioma with an alveolar adenoma-like area in the upper half (H&E stain, ×100). (G) One sclerosing hemangioma with adenocarcinoma-like area (H&E stain, ×100). |
Table 1
Clinicopathologic features of sclerosing hemangioma of the lung

SH, sclerosing hemangioma; F/U, follow-up; L, lobectomy; W, wedge resection; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; LN, lymph node; AAH, atypical adenomatous hyperplasia; P, papillary; H, hemorrhagic; Sc, sclerotic; So, solid.
*: dominant pattern in order.
References
1. Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956. 9:53–75.

2. Huszar M, Suster S, Herczeg E, Geiger B. Sclerosing hemangioma of the lung. Immunohistochemical demonstration of mesenchymal origin using antibodies to tissue-specific intermediate filaments. Cancer. 1986. 58:2422–2427.

3. Katzenstein AL, Weise DL, Fulling K, Battifora H. So-called sclerosing hemangioma of the lung. Evidence for mesothelial origin. Am J Surg Pathol. 1983. 7:3–14.
4. Xu HM, Li WH, Hou N, Zhang SG, Li HF, Wang SQ, Yu ZY, Li ZJ, Zeng MY, Zhu GM. Neuroendocrine differentiation in 32 cases of so-called sclerosing hemangioma of the lung: identified by immunohistochemical and ultrastructural study. Am J Surg Pathol. 1997. 21:1013–1022.

5. Chan AC, Chan JK. Pulmonary sclerosing hemangioma consistently expresses thyroid transcription factor-1 (TTF-1): a new clue to its histogenesis. Am J Surg Pathol. 2000. 24:1531–1536.
6. Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol. 2000. 24:906–916.
7. World Health Organization. Histological Typing of Lung Tumors. 1981. Geneva: World Health Organization.
8. Travis WD, Colby TV, Corrin B, Brambilla E. Histological Typing of Lung and Pleural Tumors. 1999. Berlin: Springer.
9. Rodriguez-Soto J, Colby TV, Rouse RV. A critical examination of the immunophenotype of pulmonary sclerosing hemangioma. Am J Surg Pathol. 2000. 24:442–450.
10. Stahlman MT, Gray ME, Whitsett JA. Expression of thyroid transcription factor-1 (TTF-1) in fetal and neonatal human lung. J Histochem Cytochem. 1996. 44:673–678.
11. Khoor A, Whitsett JA, Stahlman MT, Olson SJ, Cagle PT. Utility of surfactant protein B precursor and thyroid transcription factor 1 in differentiating adenocarcinoma of the lung from malignant mesothelioma. Hum Pathol. 1999. 30:695–700.

12. Lin D, Zou S, Lu N, Liu X, Wen P, Li L. Thyroid transcription factor-1 in the histogenesis of plumonary sclerosing hemangioma. Zhonghua Zhong Liu Za Zhi. 2002. 24:384–387.
13. Tanaka I, Inoue M, Matsui Y, Oritsu S, Akiyama O, Takemura T, Fujiwara M, Kodama T, Shimosato Y. A case of pneumocytoma (so-called sclerosing hemangioma) with lymph node metastasis. Jpn J Clin Oncol. 1986. 16:77–86.
15. Chung KY, Kim KD, Lim SH, Shin DH. A case of pneumocytoma (sclerosing hemangioma) with lymph node metastasis: a case report. Korean J Thorac Cardiovasc Surg. 1997. 30:548–551.
16. Chen CS. Inflammatory pseudotumor and lung adenoma. Chin Med J (Engl). 1978. 4:297–298.
17. Nicholson AG, Magkou C, Snead D, Vohra HA, Sheppard MN, Goldstraw P, Beddow E, Hansell DM, Travis WD, Corrin B. Unusual sclerosing haemangiomas and sclerosing haemangioma-like lesions, and the value of TTF-1 in making the diagnosis. Histopathology. 2002. 41:404–413.

18. Yano M, Yamakawa Y, Kiriyama M, Hara M, Murase T. Sclerosing hemangioma with metastases to multiple nodal stations. Ann Thorac Surg. 2002. 73:981–983.

19. Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, Colby TV. Pulmonary sclerosing hemangioma with lymph node metastases: report of 4 cases. Arch Pathol Lab Med. 2003. 127:321–325.
20. Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J. 2003. 44:150–154.

21. Niho S, Suzuki K, Yokose T, Kodama T, Nishiwaki Y, Esumi H. Monoclonality of both pale cells and cuboidal cells of sclerosing hemangioma of the lung. Am J Pathol. 1998. 152:1065–1069.
22. Hayashi A, Takamori S, Mitsuoka M, Fujimoto K, Rikimaru T, Jimi A, Shizouzu K. Unilateral progressive multiple sclerosing hemangioma in a young female successfully treated by pneumonectomy: report of a case. Int Surg. 2002. 87:69–72.
23. Katzenstein AL, Gmelich JT, Carrington CB. Sclerosing hemangioma of the lung: a clinicopathologic study of 51 cases. Am J Surg Pathol. 1980. 4:343–356.
24. Joshi K, Shankar SK, Gopinath N, Kumar R, Chopra P. Multiple sclerosing haemangiomas of the lung. Postgrad Med J. 1980. 56:50–53.

25. Noguchi M, Kodama T, Morinaga S, Shimosato Y, Saito T, Tsuboi E. Multiple sclerosing hemangiomas of the lung. Am J Surg Pathol. 1986. 10:429–435.

26. Maezato K, Hitomi S, Kuwabara M. [A case of multiple sclerosing hemangiomas of the lung and a review of the literature in Japan]. Nihon Kyobu Shikkan Gakkai Zasshi. 1989. 27:230–233.
27. Lee ST, Lee YC, Hsu CY, Lin CC. Bilateral multiple sclerosing hemangiomas of the lung. Chest. 1992. 101:572–573.

28. Leong AS, Chan KW, Seneviratne HS. A morphological and immunohistochemical study of 25 cases of so-called sclerosing haemangioma of the lung. Histopathology. 1995. 27:121–128.

29. Chon S-H, Jeon YB, Jung TY, Chung WS, Kim Y-H, Kang J-H, Jee H-O, Hong EK, Jeon S-C. Multiple sclerosing hemangiomas of the lung -a case report-. Korean J Thorac Cardiovasc Surg. 1999. 32:408–412.
30. Im JG, Kim WH, Han MC, Han YM, Chung JW, Ahn JM, Do YS. Sclerosing hemangiomas of the lung and interlobar fissures: CT findings. J Comput Assist Tomogr. 1994. 18:34–38.
31. Sakamoto K, Okita M, Kumagiri H, Kawamura S, Takeuchi K, Mikami R. Sclerosing hemangioma isolated to the mediastinum. Ann Thorac Surg. 2003. 75:1021–1023.

32. Ahmetoglu A, Kosucu P, Imamoglu M, Reis A, Cay A, Gumele HR. Sclerosing haemangioma arising within extralobar pulmonary sequestration. Pediatr Radiol. 2003. 33:641–643.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download