Abstract
The aim of present study was to establish normative data for the distribution of nuchal translucency (NT) thickness in normal Korean fetuses. The data were collected from pregnant women with singleton pregnancies in whom fetal ultrasound was performed and the fetal NT thickness was measured between 11 and 14 weeks of gestation. Among them, a total of 2,577 fetuses with a known normal outcome were included in this study. The distribution of multiple of median (MoM) values of the NT thickness with crown-rump length (CRL) in 10-mm intervals and the 95th percentile of MoM were calculated with the linear regression method. The present study showed that NT measurements increase with increasing CRL and a false positive rate increases with increasing gestational age. Therefore, a fixed cut-off point through the first trimester was not appropriate and each NT measurement should be examined according to the gestational age. The present study offers normative data of the fetal NT thickness in a Korean population, which can be used as reference for screening chromosomal aberrations or other congenital abnormalities in the first trimester.
The association between fetal chromosomal abnormality and increased nuchal translucency (NT) thickness during the first trimester of pregnancy is well-established (1, 2). Such a screening test may identify over 70% of trisomy 21 pregnancies in the first trimester, with a 5% screen-positive rate (1, 2). Even in a chromosomally normal fetus, the appearance of a thickened NT during 11-14 weeks of gestation is also strongly associated with fetal structural defects, genetic syndromes, and poor perinatal outcomes (3-5).
In the first trimester of pregnancy, NT has been widely used as a screening test for fetal Down syndrome either alone or in combination with serum markers (6). It is well documented that ethnic differences in biochemical markers levels exist both in the first and second trimesters (7, 8). A recent report showed that there was a small but significant difference in NT thickness measurements between fetuses of different ethnic origins (9). However, there have, up to now, been only limited data of NT thickness in the first trimester for Korean population (10-12). It is therefore important to establish normative data for the distribution of NT thickness. Here we report our results of ultrasound measurement of NT thickness in 2,577 normal fetuses between 11 and 14 weeks of gestation in a Korean population.
The data were collected from pregnant women with singleton pregnancies in whom fetal ultrasound was performed and the fetal NT thickness was measured between 11 and 14 weeks of gestation at our institute during the period from January 2001 to December 2001. Pregnancy outcomes were reviewed from the obstetric and neonatal records. Those cases with no obstetrical or neonatal records were excluded from analysis. Only cases with a known normal outcome which could be found in Samsung Cheil Hospital Obstetrics Database were included. Cases affected by chromosomal and major structural abnormalities and cases resulting in miscarriage or intrauterine death were excluded from analysis.
The gestational age was calculated from the first day of the last menstrual period and was confirmed by crown-rump length measurement (13). In cases in which the estimated gestational age by menstrual and ultrasound estimation were discrepant for more than 7 days, the ultrasound estimation was used.
Fetal NT thickness was measured using transabdominal or transvaginal sonography (HDI 3000, ATL, Bothell, WA, U.S.A.) in a good mid-sagittal section of the fetus with magnification so that the fetus occupied at least 75% of the image. The NT was defined as the black area between the inner skin outlines echo and the outer border of the soft tissue overlying the cervical spine. The maximal thickness of the black area was measured with caliper placed on the lines (representing the nuchal skin and the underlying soft tissue) to 0.1 mm when the sagittal section of the fetuses was obtained (Fig. 1). At the same time, the fetal crown-rump length (CRL) was measured. Cystic hygroma was defined as a sonolucent area consisting of two systemic cavities completely separated by a midline septum, irrespective of size (14). These cases were excluded from the study. Care was taken not to take a measurement with the fetus overextended because an extended fetal neck will result in a falsely increased measurement (15). Care was also taken to distinguish between the fetal skin and the amniotic membrane. If it was impossible to obtain a proper image of the fetal nuchal translucency due to fetal position, we waited for spontaneous fetal movement away from the amniotic membrane; alternatively, the fetus was bounced off amnion by asking the mother to cough and/or tapping the maternal abdomen. In such cases it was helpful to use the cineloop function (a function that permits the replay of ultrasound images taken in the past few seconds) to visualize the fetus in a satisfactory position. At least three measurements were taken during the scan and the largest was recorded.
In order to establish normative data of NT thickness, the relationship between fetal NT thickness and CRL was analyzed with the linear regression method. According to the regression equation, the expected the 5th, 50th, and 95th percentile values of NT thickness were obtained for a given CRL.
All the cases were subdivided into five categories by the CRL of the fetuses with 10-mm intervals. To correct for the gestational effect, the median value of NT thickness in each CRL category was calculated and then all the measurements of NT thickness were converted to multiple of median (MoM) values.
Statistical analysis was performed by Statistics Package for Social Science (SPSS) 10.0 computer software. A p value of <0.05 was considered statistically significant.
Between January and December 2001, a total of 2,577 pregnancies satisfied the above inclusion criteria and were included for analysis. The mean maternal age was 29.94±3.28 yr (range 19-44 yr). The mean CRL was 60.16±9.07 mm (range 40.0-92.0 mm), the mean NT thickness was 1.62±0.50 mm (range 0.5-5.0 mm), respectively. The median gestational age was 12.5 weeks.
Fig. 2 plots the distribution and the 5% and 95%, lower and upper limits of NT thickness according to the simple linear regression. NT thickness increased with CRL estimates of gestational age. The regression equation relating median NT thickness to CRL was described as follows: expected NT thickness (mm)=0.437+0.01969×CRL (mm) (R2=0.127, p<0.001).
The expected 5th, 50th, and 95th percentile values of NT thickness to CRL are listed in Table 1. The median NT thickness increased from 1.22 mm when the CRL measurement was 40 mm to 2.11 mm with 85 mm sized CRL.
Table 2 shows the distribution of the NT thickness with 10-mm intervals of CRL. The mean NT MoM value to 10-mm intervals of CRL was 0.998±0.298, and the 95th percentile of NT MoM was 1.50.
Table 3 shows the distribution of the NT thickness with gestational age.
The incidence of NT thickness greater than or equal to 2.5 mm was increased; 2.2% in fetuses at 11.0-11.9 weeks of gestation to 12.5% in fetuses at 14.0-14.9 weeks. One-hundred and three out of the 2,577 fetuses (4.0%) had NT thickness greater than or equal to 2.5 mm.
Our results demonstrate that fetal NT thickness increases with CRL and are compatible with those of previous reports (4, 16, 17). Therefore, in screening for chromosomal defects, the use for a fixed cut-off in fetal NT thickness is inappropriate, and each measurement should be examined according to the CRL (18). Schuchter et al. have used MoM values to express the relationship between NT measurement and gestational age (19). This method is similar to that of biochemical markers used in second-trimester Down screening (20). Other studies asserted that the more accepted method is to base the cut-off on a progressive rise, using 95th percentile as the threshold for an abnormal NT thickness, resulting in a more sensitive and specific indicator for the detection of anomalous fetuses (21). Therefore, it is mandatory to establish the normative distribution of fetal NT measurement.
Several studies have been reported on the value of NT thickness in the first trimester in Korean population (22-24). These studies, however, are limited as they used a fixed cut-off in fetal NT thickness. More recently, several other studies have been reported in Korean literature (10-12), but they have relatively small sample size. The present study includes a very large number of pregnant women for analysis and thus offers normative data of the fetal NT thickness in a Korean population. Compared with the recent study by Jou et al. (25), which measured the NT thickness from 897 Taiwanese pregnancies between 9-14 gestational weeks, our results was similar to those.
There is a controversy as to the need to take the ethnic difference into account in the interpretation of NT measurements. Jou et al. suggested that, given the small but statistically significant differences, race-specific normative data should be used (25). Other authors concluded that it is acceptable to use a single standard, because screen positive rates in different groups are similar (9, 26). In our study, the mean NT thickness in normal Korean fetuses was 1.62 mm. Compared to the study in Taiwanese population by Jou et al. (25), mean NT thickness in our result was smaller than those by 0.1 mm. Otherwise, compared with the study in Caucasians by Thilaganathan et al. (9), the difference was rather small (0.08 mm). Therefore, we suggest that the ethnic difference is not significant in interpretation of NT measurements.
In our study, the incidence of NT thickness greater than or equal to 2.5 mm was 4.0%. This observation is in accordance with previous studies (2, 17). Scott et al. reported that the incidence of NT thickness greater than or equal to 2.5 mm in normal fetuses increased; 1.3% at CRL 30-39 mm to 13.2% at CRL 60-69 mm (17). In our study, the incidence of NT thickness greater than or equal to 2.5 mm in normal fetuses was 2.2% at 11.0-11.9 weeks of gestation and increased to 12.5% at 14.0-14.9 weeks. Therefore, we confirm that a false positive rate increases with increasing gestational age, and each NT measurement should be examined according to the gestational age for screening of chromosomal abnormalities.
More recently, a new ultrasound marker has been described; the nasal bone at 11 to 14 weeks was found to be absent in about 70% of fetuses with trisomy 21 and in 0.5% of chromosomally normal fetuses. It was estimated that screening for trisomy 21 by a combination of maternal age, fetal NT thickness, and examination of the nasal bone could increase the detection rate to 85% whilst decreasing the false-positive rate to 1% (27). Also, Cicero et al. reported that integrated sonographic (fetal NT thickness and nasal bone) and biochemical test using maternal serum free β-hCG and pregnancy-associated plasma protein-A (PAPPA) at 11-14 gestational weeks can potentially identify about 90% of trisomy 21 fetuses for a false-positive rate of 0.5% (28). We are planning to investigate the diagnostic application of these integrated approach in Korean fetuses at 11-14 gestational weeks to screen of trisomy 21 in the near future.
The present study offers normative data of the fetal NT thickness in a Korean population, which can be used as reference for screening chromosomal aberrations or other congenital abnormalities and may be very helpful in establishing the screening for Down syndrome in the first trimester in Korea. As a false positive rate increases with increasing gestational age, NT measurement should be adjusted according to the gestational age for screening of chromosomal abnormalities.
Figures and Tables
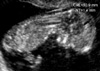 | Fig. 1Measurement of the nuchal translucency (NT) thickness on transvaginal ultrasound scan in 12.0 weeks sized fetus. |
 | Fig. 2The distribution of nuchal translucency (NT) thickness in normal fetuses with crown-rump length. |
Table 1
The expected 5th, 50th, and 95th percentile values of nuchal translucency (NT) with crown-rump length (CRL)

References
1. Nicolaides KH, Brizot ML, Snijders RJ. Fetal nuchal translucency: ultrasound screening for fetal trisomy in the first trimester of pregnancy. Br J Obstet Gynaecol. 1994. 101:782–786.

2. Pandya PP, Snijders RJ, Johnson SP, De Lourdes Brizot M, Nicolaides KH. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to14 weeks of gestation. Br J Obstet Gynaecol. 1995. 102:957–962.
3. Pandya PP, Kondylios A, Hilbert L, Snijders RJ, Nicolaides KH. Chromosomal defects and outcome in 1015 fetuses with increased nuchal translucency. Ultrasound Obstet Gynecol. 1995. 5:15–19.

4. Hyett J, Perdu M, Sharland G, Snijders R, Nicolaides KH. Using fetal nuchal translucency to screen for major congenital cardiac defects at 10-14 weeks of gestation: population based cohort study. Br Med J. 1999. 318:81–85.
5. Hafner E, Schuchter K, Liebhart E, Philipp K. Results of routine fetal nuchal translucency measurement at weeks 10-13 in 4233 unselected pregnant women. Prenat Diagn. 1998. 18:29–34.

6. Spencer K, Spencer CE, Power M, Moakes A, Nicolaides KH. One stop clinic for assessment of risk for fetal anomalies: a report of the first year of prospective screening for chromosomal anomalies in the first trimester. BJOG. 2000. 107:1271–1275.

7. Watt HC, Wald NJ, Smith D, Kennard A, Densem J. Effect of allowing for ethnic group in prenatal screening for Down's syndrome. Prenat Diagn. 1996. 16:691–698.

8. Spencer K, Ong CY, Liao AW, Nicolaides KH. The influence of ethnic origin on first trimester biochemical markers of chromosomal abnormalities. Prenat Diagn. 2000. 20:491–494.

9. Thilaganathan B, Khare M, Williams B, Wathen NC. Influence of ethnic origin on nuchal translucency screening for Down's syndrome. Ultrasound Obstet Gynecol. 1998. 12:112–114.

10. Park JW, Lee WK. Changes of nuchal translucency in early normal fetuses. Korean J Obstet Gynecol. 2000. 43:998–1001.
11. Lee K, Cha DH, Park SP, Park HJ. Nuchal translucency measurement in normal fetuses at 10-14 weeks of gestation I. Korean J Obstet Gynecol. 2000. 43:1822–1827.
12. Lee K, Cha DH, Kim JH, Seo SK, Lee DW, Cho SH, Kwon JY. Nuchal translucency measurement in normal Korean fetuses at 10-14 weeks of gestation (II). Korean J Obstet Gynecol. 2003. 46:522–527.
13. Hadlock FP, Shah YP, Kanon DJ, Lindsey JV. Fetal crown-rump length: Reevaluation of relation to menstrual age (5-18 weeks) with high-resolutin real-time US. Radiology. 1992. 182:501–505.
14. Ville Y, Lalondrelle C, Doumerc S, Daffos F, Frydman R, Oury JF, Dumez Y. First trimester diagnosis of nuchal anomalies: significance and fetal outcome. Ultrasound Obstet Gynecol. 1992. 2:314–316.
15. Whitlow BJ, Chatzipapas IK, Economides DL. The effect of fetal neck position on nuchal translucency measurement. Br J Obstet Gynaecol. 1998. 105:872–876.

16. Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation. Lancet. 1998. 352:343–346.

17. Scott F, Boogert A, Sinosich M, Anderson J. Establishment and application of a normal range for nuchal translucency across the first trimester. Prenat Diagn. 1996. 16:629–634.

18. Taipale P, Hiilesmaa V, Salonen R, Ylostalo P. Increased nuchal translucency as a marker for fetal chromosomal defects. N Engl J Med. 1997. 337:1654–1658.

19. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10-13 weeks of pregnancy. Prenat Diagn. 1998. 18:281–286.

20. Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, Haddow JE, Knight GJ, Palomaki GE, Canick JA. Maternal serum screening for Down's syndrome in early pregnancy. Br Med J. 1988. 297:883–887.

21. Snijders RJ, Johnson S, Sebire NJ, Noble PL, Nicolaides KH. First-trimester ultrasound screening for chromosomal defects. Ultrasound Obstet Gynecol. 1996. 7:216–226.

22. Kim MY, Ryu HM, Kim ES, Han HW, Yang JH, Yoo SJ, Lee YH, Han JR, Lee KS. The value of increased nuchal translucency (NT) for the prediction of abnormal pregnancy outcome. Korean J Perinatol. 1998. 9:363–374.
23. Lee JY, Choi KH, Park CW, Yun TS, Park CJ, Jang PR, Park YS. Fetal nuchal translucency measurement for detection of chromosomal abnormalities in the first trimester of high risk pregnancy. Korean J Obstet Gynecol. 1998. 41:2739–2742.
24. Kim SJ, Kim CM, Min BS, Sohn WS, Kang JB, Jang PR. The efficacy of nuchal translucency with free beta-hCG, PAPP-A as a screening test for detection of chromosomal anomaly in the first trimester of pregnancy. Korean J Obstet Gynecol. 2001. 44:1091–1096.
25. Jou HJ, Wu SC, Li TC, Hsu HC, Tzeng CY, Hsieh FJ. Relationship between fetal nuchal translucency and crown-rump length in an Asian population. Ultrasound Obstet Gynecol. 2001. 17:111–114.

26. Chen M, Lam YH, Tang MH, Lee CP, Sin SY, Tang R, Wong HS, Wong SF. The effect of ethnic origin on nuchal translucency at 10-14 weeks of gestation. Prenat Diagn. 2002. 22:576–578.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download