Abstract
Acquired perforating dermatosis (APD) is a skin disorder occurring in the patients with chronic renal failure (CRF), diabetes mellitus (DM) or both. The purpose of this study was to clarify the clinical and histopathological features of APD, and evaluate role of scratching in the pathogenesis of APD. Twelves patients with APD associated with CRF and DM were enrolled in the study. In six patients who required hemodialysis, the lesions appeared 2-5 yr (mean 3 yr) after the initiation of dialysis, 18-22 yr (mean 19.3 yr) after the occurrence of DM. The other patients who did not receive hemodialysis noted the lesions 4-17 yr (mean 9.5 yr) after the onset of DM. All patients had an eruption of generally pruritic keratotic papules and nodules, primarily on the extensor surface of the extremities and the trunk. The histologic features of our cases showed a crateriform invagination of the epidermis filled by a parakeratotic plug and basophilic cellular debris. The period of treatment for patients who suffered from severe (7 cases) or very severe (3 cases) on the pruritus intensity was longer than that of patients who had mild pruritus (2 cases). These data showed that scratching appear to play a critical part in the pathogenesis of APD.
The perforating disorders of the skin are a group of diseases characterized by transepidermal elimination of material from the upper dermis and are classified histopathologically according to the type of epidermal disruption and the nature of the eliminated material. An acquired perforating dermatosis (APD) has recently become a well recognized association of chronic renal failure and is most commonly seen where the underlying cause of renal failure is diabetic nephropathy and in patients undergoing hemodialysis (1). Although the pathogenesis of APD is not known, some authors suggest that trauma (scratching) and microvasculopathy are triggers of transepidermal elimination and degeneration of collagen fibers (2).
The purpose of the present study was to clarify the clinical and histopathological features of APD, and evaluate role of scratching in the pathogenesis of APD and relation between intensity of pruritus and duration of treatment.
Twelves patients with APD associated with chronic renal failure and diabetes mellitus were enrolled in the study. All patients were consulted from Department of Internal Medicine (Aug 1996-June 2003). APD was diagnosed on the basis of following clinical and histopathologic setting: (1) histopathological findings of transepidermal elimination of basophilic cellular debris into invagination of the epidermis; (2) pruritic keratotic umblicated papules or nodules.
All tissue specimens were obtained by skin biopsy, fixed in 10% formalin, step-sectioned and stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), Verhoeff-van Gieson and Masson trichrome stains.
Patients self-assessed pruritus severity in a daily diary before treatment. Pruritus severity was rated on a five-point scale (none to vere severe) (Table 1). The patients were cautioned against scratching around the cutaneous lesion, and this area was protected with gauze and bandage. Patients were treated with anti-histamines, tranquillizers, hypnotics, and phototherapy ultra violet B (UVB) for clearing pruritus.
The clinical features of our patients are shown in Table 2. There were eight men and four women. Their age at the time of initial examination ranged from 43 to 66 yr (mean 58 yr). Also APD duration ranged from 1 months to 24 months (mean 5.8 months). Ten patients had non-insulin-dependent diabetes mellitus, and two patient had insulin-dependent diabetes mellitus. In six patients who required hemodialysis, the lesions appeared 2-5 yr (mean 3 yr) after the initiation of dialysis, 18-22 yr (mean 19.3 yr) after the occurrence of diabetes mellitus. The other patients who did not have hemodialysis noted the lesions 4-17 yr (mean 9.5 yr) after the onset of diabetes mellitus. A large number of patients had diabetic vasculopathy such as retinopathy, nephropathy, gangrene and neuropathy.
All patients had an eruption of generally pruritic keratotic papules and nodules, primarily on the extensor surface of the extremities and the trunk. The lesions typically had a central keratin-filled crater and might be grouped to form small plaques or arranged in a linear fashion (Koebner phenomenon) (Fig. 1).
The histologic features of our ten cases showed a vertically oriented, shallow, cup-shaped invagination of the epidermis, forming a short channel. The channel was lined by acanthotic epithelium along the sides. At the base of the invagination there was an attenuated layer of keratinocytes that in some foci appear eroded. Within the channel there was densely packed degenerated basophilic staining material, neutrophils, and basophilically altered collagen bundles. Vertically oriented perforating bundles of collagen were present interposed between the keratinocytes of the attenuated bases of the invagination. Masson trichrome staining showed transepidermal elimination of the collagen (Fig. 2). Verhoeff-van Gieson staining for elastic fibers was negative in the epidermis and in the crater. The overall histologic appearances were consistent with a reactive perforating collagenosis. And the histologic features of two cases showed central epidermal invagination filled with heavy keratotic, partly parakeratotic plug and basophilic debries. Many vacuolated cells and a few dyskeratotic cells were seen below the keratotic plug (Fig. 3). Masson's trichrome and Verhoeff-van Gieson stains was negative in the epidermis and in the crater. The overall histologic appearances were consistent with a Kyrle's disease. In eight cases, the dermis below the crater contained numerous vessels, many of which had a thickened and PAS-positive wall and a mixed infiltrate of mononuclear cells and neutrophils with occasional eosinophils (Fig. 4).
At presentation, the majority of patients suffered from severe (7 cases) to very severe (3 cases) pruritus. Only two cases had mild or moderate pruritus. In nine cases, the number of lesions decreased and disappeared within a few months, leaving some degree of scarring and pigmentation. However, three cases showed no improvement of lesions despite of treatment. The duration of treatment ranged from 6 weeks to 25 weeks (mean 13.9 weeks). The period of treatment for patients who suffered from severe or vere severe on the pruritus intensity was longer than that of patients who had mild pruritus (Table 3).
The perforating disorders of the skin are characterized by the transepidermal elimination of some component of the dermis and have classically been divided into four types: elastosis perforans serpiginosa (EPS), reactive perforating collagenosis (RPC), perforating folliculitis (PF) and Kyrle's disease (KD). All four of these disorders have been described in patients with chronic renal failure (CRF), diabetes mellitus (DM), or both. Although early reports describe the perforating disorders seen in association with these disease states as reactive perforating collagenosis (3, 4), there is now a consensus that perforating disorders secondary to CRF, DM, or both, represent a separate dermatosis (5, 6). Rapini et al. (5) suggested the name "acquired perforating dermatosis," which seems quite appropriate for this condition. The name avoids describing what material is being transepidermally eliminated, which is significant as cases of APD often show perforation of both collagen and elatic fibers. Furthermore, the name encompasses the broad spectrum of perforating disorders of CRF, DM, or both, since the varying histologic findings probably represent different stages or different types of lesions in the same pathologic process (5).
The lesions of APD associated with CRF, DM, or both, are 2 to 10 mm, firm, hyperkeratotic, often umbilicated papules usually occurring on the extremities, especially on the legs. The lesions are often pruritic, and Koebner's phenomenon may occur after scratching, rubbing, or trauma to the lesion (7). In our study, all patients had an eruption of generally pruritic keratotic papules and nodules. The most lesions had a central keratin-filled crater and may be grouped to form small plaques or arranged in a linear fashion. However, the most frequently involved sites were trunk, upper extremities, lower extremities, and buttock in descending order. Unusual present sites such as face, hand and foot (each of one patient) were also involved in our study.
The majority of patients with APD have CRF, DM, or both (5, 7, 8). Morton et al. (9) have found that, of 72 patients attending a British dialysis unit (48 on hemodialysis, 24 peritoneal dialysis), eight (all whites) had APD. This prevalence of 11% is similar to the 4.5-10% reported for North American dialysis patients, among whom was a high proportion of black patients. In the study of Morton et al. (9), APD had developed before dialysis in two patients, at the start of hemodialysis in two, at the start of peritoneal dialysis in one, and 1-3 yr after start of peritoneal dialysis in three patients. Seven of the APD patients had insulin-dependent DM, median duration 20 yr (range 16-37) and four had non-insulin-dependent DM (duration 2, 10, 11, and 22 yr). However, APD has been reported in non-insulin-dependent DM from other centers (10). In our study, ten patients had non-insulin-dependent DM, and two patient had insulin-dependent DM. In six patients who required hemodialysis, the lesions appeared 2-5 yr (mean 3 yr) after the initiation of dialysis, 18-22 yr (mean 19.3 yr) after the occurrence of DM. The other patients who did not have hemodialysis noted the lesions 4-17 yr (mean 9.5 yr) after the onset of DM.
The pathogenesis of APD is unknown. In our patients, APD tended to be distributed on trauma-prone areas, the dorsum of the extremities, and was often associated with superficial trauma such as abrasions and scratches. The Koebner phenomenon was evident in most cases. Pruritus was a common factor in all cases. When the patients were cautioned against scratching around the cutaneous lesion and treated with antihistamines, tranquillizers, hypnotics, and phototherapy (UVB) for clearing pruritus, the number of lesions decreased and disappeared within a few months. The period of treatment for patients who suffered from severe or very severe on the pruritus intensity was longer than that of patients who had mild pruritus. Many researchers have suggested that APD represents a cutaneous response to superficial trauma (11-13). We regard the trauma as a specific reaction caused by scratching in association with DM. These superficial reactions appear to play a critical part in the pathogenesis of APD. In particular, DM patients report a high incidence of pruritus (14). A large number of APD patients also have DM, suggesting that diabetic vasculopathy is an underlying factor in this eruption (3, 4, 15). Retinopathy, nephropathy, gangrene and neuropathy in our patients were suggestive of diabetic microangiopathy. Histopathologically, we found PAS-positive thickening of the vessel walls in the upper dermis. Based on these findings, one theory concerns the effect of diabetic vasculopathy combined with the trauma (pruritus), in DM patients and often also experienced by nephropathy patients (14, 16). That is, trauma from scratching may cause dermal necrosis due to the poor blood supply, with the necrotic dermal material then extruded through the epidermis. Furthermore, in our study, the eruptions tended to appear a relatively long time after the initial diagnosis of DM, but were unrelated to dialysis. Most patients had non-insulin-dependent DM. Although Morton et al. (9) reported APD more often in insulin-dependent DM compared with non-insulin-dependent DM, APD has been more strongly associated with non-insulin-dependent DM in other reports (10, 15). Thus, based on the present findings as well as previous reports, DM type does not appear to be a predisposing factor in APD.
An alternative hypothesis is that the extrusion of dermal contents is a form of foreign-body reaction to some constituents of the dermis. Experimental support for this view comes from reports that introduction of charcoal particles into the skin of guinea pigs results in dermal hyperplasia and transepidermal elimination (17). Despite suggestions that the collagen is eliminated because it is abnormal, there is no consistent morphological evidence for collagen defects in the dermis below early lesions. However, it is possible that the collagen might have been biochemically but not morphologically altered. The ultrastructural alteration in elastic fibers at the base of early lesions might also be recognized as foreign material (18).
APD has also been reported in other cases of renal impairment not due to diabetes, including obstructive nephropathy, hypertensive nephrosclerosis, acquired immune deficiency syndrome, chronic nephritis and anuria (10, 19-21). This suggests that the skin changes in CRF itself may be of importance in the development of APD. Dermal microdeposition of substances such as calcium salt may promote a local inflammatory reaction and connective tissue degradation (22). Certainly crystal-like micro-deposits in the upper dermis have been observed in one ultrastructural study of APD (23).
On the basis of our study, intensity of pruritus seems to be related to the duration of treatment. Thus it is reasonable to assume that scratching is involved in pathogenesis of APD and trauma and microvasculopathy are triggers of transepidermal elimination and degeneration of collagen fibers. However, little is known about the origin of this altered collagen and thus we think further study is needed in the future.
Figures and Tables
Fig. 1
Linear hyperkeratotic umbilicated papules on the extensor surface of the extremities and the trunk (A, B, C, D). Note Koebner phenomenon from scratching.
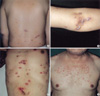
Fig. 2
Collagen fibers with near-vertical extrusion into keratinous epidermal depression (Masson trichrome stain, ×200).

Fig. 3
Kyrle's disease showing a heavy keratotic, partly parakeratotic plug containing basophilic debris lies in an invagination of the epidermis (H&E, ×40).

References
1. Maurice PD. Acquired perforating dermatosis in renal patients. Nephrol Dial Transplant. 1997. 12:2774–2775.

2. Kawakami T, Saito R. Acquired reactive perforating collagenosis associated with diabetes mellitus: eight cases that meet Faver's criteria. Br J Dermatol. 1999. 140:521–524.

3. Poliak SC, Lebwohl MG, Parris A, Prioleau PG. Reactive perforating collagenosis associated with diabetes mellitus. N Engl J Med. 1982. 306:81–84.

4. Cochran RJ, Tucker SB, Wilkin JK. Reactive perforating collagenosis of diabetes mellitus and renal failure. Cutis. 1983. 31:55–58.
5. Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. Evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989. 125:1074–1078.

6. Heilman ER, Friedman RJ. Elder D, editor. Lever's Histopathology of the Skin. 1997. Philadelphia: Lippincott-Raven;341–351.
7. Lee MW, Choi JH, Sung KJ, Moon KC, Koh JK. Acquired perforating dermatosis in renal failure. Korean J Dermatol. 2000. 38:1157–1161.
8. Bang SW, Bu TS, Hyun C, Whang KU, Kim YK. Four cases of acquired perforating disease in patients with chronic renal failure. Korean J Dermatol. 1997. 35:333–338.
9. Morton CA, Henderson IS, Jones MC, Lowe JG. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996. 135:671–677.

10. Faver IR, Daoud MS, Su WP. Acquired reactive perforating collagenosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994. 30:575–580.
11. Pedragosa R, Knobel HJ, Huguet P, Oristrell J, Valdes M, Bosch JA. Reactive perforating collagenosis with Hodgkin's disease. Am J Dermatopathol. 1987. 9:41–44.
12. Mehregan AH. Elastosis perforans serpiginosa: a review of the literature and report of 11 cases. Arch Dermatol. 1968. 97:381–393.

13. Yuzuk S, Trau H, Stempler D, Sofer E, Levy A, Schewach-Millet M. Reactive perforating collagenosis. Int J Dermatol. 1985. 24:584–586.

15. Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989. 20:287–289.

17. Bayoumi AH, Gaskell S, Marks R. Development of a model for transepidermal elimination. Br J Dermatol. 1978. 99:611–620.

18. Patterson JW, Brown PC. Ultrastructural changes in acquired perforating dermatosis. Int J Dermatol. 1992. 31:201–205.

19. Hood A, Hardegen GL, Zarate AR, Nigra TP, Gelfand MC. Kyrle's disease in patients with chronic renal failure. Arch Dermatol. 1982. 118:85–88.

20. Bank DE, Cohen PR, Kohn SR. Reactive perforating collagenosis in a setting of double disaster: acquired immunodeficiency syndrome and end-stage renal disease. J Am Acad Dermatol. 1989. 21:371–374.

21. Stone RA. Kyrle-like lesions in two patients with renal failure undergoing dialysis. J Am Acad Dermatol. 1981. 5:707–709.

22. Blanchley JD, Blankenship M, Menter A. Uremic pruritus: skin divalent ion content and response to ultraviolet therapy. Am J Kidney Dis. 1985. 5:237–241.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download