1. Chan JK. Mesenchymal tumors of the gastrointestinal tract: a paradise for acronyms (STUMP, GIST, GANT, and now GIPACT), implication of c-kit in genesis, and yet another of the many emerging roles of the interstitial cell of Cajal in the pathogenesis of gastrointestinal diseases? Adv Anat Pathol. 1999. 6:19–40.

2. Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998. 152:1259–1269.
3. Eyden B, Chorneyko KA, Shanks JH, Menasce LP, Banerjee SS. Contribution of electron microscopy to understanding cellular differentiation in mesenchymal tumors of the gastrointestinal tract: a study of 82 tumors. Ultrastruct Pathol. 2002. 26:269–285.

4. Yantiss RK, Rosenberg AE, Selig MK, Nielsen GP. Gastrointestinal stromal tumors: an ultrastructural study. Int J Surg Pathol. 2002. 10:101–113.

5. Liu P, Na J, Wang Y, He Q, Zhang Y, Tang X, Zou W. Study of gastrointestinal stromal tumors by light microscopy, electron microscopy and immunohistochemistry. Zhonghua Bing Li Xue Za Zhi. 2002. 31:199–203.
6. Dhimes P, Lopez-Carreira M, Ortega-Serrano MP, Garcia-Munoz H, Martinez-Gonzalez MA, Ballestin C. Gastrointestinal autonomic nerve tumours and their separation from other gastrointestinal stromal tumours: an ultrastructural and immunohistochemical study of seven cases. Virchows Arch. 1995. 426:27–35.

7. Matsumoto K, Min W, Yamada N, Asano G. Gastrointestinal autonomic nerve tumors: immunohistochemical and ultrastructural studies in cases of gastrointestinal stromal tumor. Pathol Int. 1997. 47:308–314.

8. Erlandson RA, Klimstra DS, Woodruff JM. Subclassification of gastrointestinal stromal tumors based on evaluation by electron microscopy and immunohistochemistry. Ultrastruct Pathol. 1996. 20:373–393.

9. Montgomery E, Torbenson MS, Kaushal M, Fisher C, Abraham SC. Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol. 2002. 26:1296–1301.
10. Makhlouf HR, Remotti HE, Ishak KG. Expression of KIT (CD117) in angiomyolipoma. Am J Surg Pathol. 2002. 26:493–497.

11. Leroy X, Aubert S, Leteurtre E, Gosselin B. Expression of CD117 in a malignant peripheral nerve sheath tumour arising in a patient with type 1 neurofibromatosis. Histopathology. 2003. 42:511–513.

12. Miettinen M, El-Rifai W, Sobin HL, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002. 33:478–483.

13. Mazur MT, Clark HB. Gastric stromal tumors: reappraisal of histogenesis. Am J Surg Pathol. 1983. 7:507–519.

14. Ma CK, Amin MB, Kintanar E, Linden MD, Zarbo RJ. Immunohistologic characterization of gastrointestinal stromal tumors: a study of 82 cases compared with 11 cases of leiomyomas. Mod Pathol. 1993. 6:139–144.
15. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002. 33:459–465.

16. Damiani S, Pasquinelli G, Eusebi V. GANT-like gastrointestinal pacemaker cell tumours with oncocytic features. Virchows Arch. 1999. 435:143–150.

17. Al-Bosom IA. p53 expression in gastrointestinal stromal tumors. Pathol Int. 2001. 51:519–523.

18. Seidal T, Edvardsson H. Expression of c-kit (CD117) and Ki67 provides information about the possible cell of origin and clinical course of gastrointestinal stromal tumours. Histopathology. 1999. 34:416–424.

19. Cunningham RE, Abbondanzo SL, Chu WS, Emory TS, Sobin LH, O'Leary TJ. Apoptosis, bcl-2 expression, and p53 expression in gastrointestinal stromal/smooth muscle tumors. Appl Immunohistochem Mol Morphol. 2001. 9:19–23.

20. Faussone-Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Microsc Res Tech. 1999. 47:248–266.

21. Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the interstitial cells of Cajal of the human stomach. J Submicrosc Cytol Pathol. 1989. 21:439–460.
22. Min KW, Seo IS. Intestitial cells of Cajal in the human small intestine: immunochemical and ultrastructural study. Ultrastruct Pathol. 2003. 27:67–78.

23. Lee JR, Joshi V, Griffin JW Jr, Lasota J, Miettinen M. Gastrointestinal autonomic nerve tumor: immunohistochemical and molecular identity with gastrointestinal stromal tumor. Am J Surg Pathol. 2001. 25:979–987.
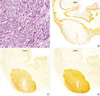
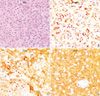
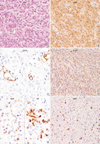
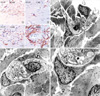
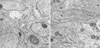
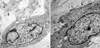
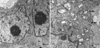






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download