Abstract
Chronic granulomatous disease (CGD) is a fatal genetic disorder in which phagocytes fail to produce antimicrobial superoxide because of NADPH oxidase deficiency. Molecular defects in CYBB gene causing X-linked CGD are responsible for about 70% of all cases. This study was done to confirm genetic defects of CYBB gene in five Korean patients who were highly suggestive of having CGD by clinical history. We performed initial screening for five unrelated Korean patients using single strand conformation polymorphism (SSCP) and then selective sequencing for the regions involving the abnormal bands. Activated NBT tests revealed that all patients were X-linked. SSCP analysis for CYBB gene showed abnormal bands in all patients. The molecular defects of five patients were as follows: c.1663insT, c.1111-1G>T, c.39_40insG, c.927delC and c.434T>C mutation. This result will help the families with prenatal diagnosis or genetic counseling.
Chronic granulomatous disease (CGD, OMIM No. 306400) is a disorder of phagocytes due to the defective activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that catalyzes the production of antimicrobial superoxide. CGD is caused by mutations in any of 4 structural components of this enzyme, including gp91-phox, p22-phox, p47-phox and p67-phox. CGD is inherited either in an X-linked or autosomal recessive manner. However, X-linked CGD is far more common, accounting for about 70% of all CGD cases (1, 2). The X-linked CGD gene, CYBB (GenBank Accession No. AF469757-AF469769), is localized on chromosome Xp21.1, spans 30 kb and contains 13 exons. Over 300 CYBB mutations have been found along the whole gene and registered in an internationally maintained X-linked CGD database (3). This study aimed to investigate the molecular characterization of 5 unrelated Korean patients with X-linked CGD. Mutations in these patients were screened initially by single strand conformation polymorphism (SSCP) and then confirmed by selective DNA sequencing of the abnormal bands.
Nitroblue tetrazolium (NBT) slide test with phorbol myristate acetate (PMA) was performed on the neutrophils of the 5 patients and their mothers. We considered a patient to have X-linked CGD when the patient showed no formazan cells while his mother showed mosaic pattern (4).
Polymerase chain reaction (PCR) and single-strand conformation polymorphism (SSCP) was done as previously described (5, 6). A few of the previously described primers sequences are slightly modified. Sequencing reaction was done using Model Prism 377 (Applied Biosystems Inc., CA, U.S.A.) and ABI Prism Dye Terminator cycle Sequencing Ready Reaction Kit (Perkin-Elmer, CA, U.S.A.). In case of splice-site mutation, CYBB gene mRNA was reversely transcribed and amplified into three overlapping fragments using three sets of synthetic primers described by Dinauer et al. (7).
A total of five Korean unrelated patients were included in this study. Patient 1 had suffered from recurrent anal abscesses and inguinal lymphadenopathy from the age of 4 months to his death at the age of two. Many bacteria were isolated from anal pus, including Enterobacter cloacae, Klebsiellla pneumoniae, Escherichia coli, Enterococcus faecium, and Enterobacter asburiae. He was finally hospitalized for inguinal lymphadenopathy and developed pneumonia, sepsis, disseminated intravascular coagulation, acute renal failure, and hepatitis. He died of septic shock. Burkholderi cepacia was isolated from blood at his death.
Patient 2 was a 12-yr-old boy who had suffered from many infectious diseases since 2 months after birth, including recurrent subcutaneous abscesses caused by K. pneumoniae, Enterobacter aerogenes, and Serratia marcescens, enteric fever by group B Salmonella spp., pneumonia by Haemophilus influenzae and Staphylococcus aureus, liver abscess by S. aureus. He had also suffered lymphadenopathy, osteomyelitis, otitis media (the causative microorganisms were not isolated), and Guillain-Barre syndrome. There had been bacteremia caused by S. epidermidis and bacteriuria by E. coli.
Patient 3 was a 22-yr-old man who had suffered from tuberculosis, lymphadenitis, hepatitis, enteric fever, meningitis, and Behçet's disease since he was 6 yr old. The following bacterial species were isolated: group B Salmonella spp. in his blood, liver biopsy and stool; coagulase negative Staphylococcus in bile; and Enterococcus in urine.
Patient 4 had suffered from recurrent lymphadenitis since six years old and suffered salmonellosis at seven. He was treated with IFN-γ. When he was ten years old, he was hospitalized for an intractable cough and rhinorrhea and aggravated to pneumonia with pleural effusion, hepatitis, acute renal failure, and died of septic shock. B. cepacia was isolated from his blood.
Patient 5 was a six-year-old boy who had suffered from upper respiratory infection, lymphadenitis and liver abscesses since four years old. No bacterial organism had been isolated. He was in good health at the time of this study.
Activated NBT slide tests showed no respiratory burst in all patients except patient 5 who showed microgranular pattern (Table 1). The cells of the mothers of patients showed a mosaic pattern. These findings are compatible with an X-linked defect in these families.
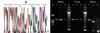
Because the defect in these patients was considered X-linked, we searched for mutations in CYBB gene. Abnormal bands by SSCP were found in all patients from exon 13, 11, 1, 9, and 5, respectively. Five patients were confirmed to have molecular defects in CYBB gene by selective sequencing of the regions (Table 1). The molecular defects in 5 patients were as follows: c.1663insT (Fig. 1), c.1111-1G>T (Fig. 2), c.39_40insG, c.927delC and c.434T>C mutation (Fig. 3).
This paper reports the biochemical and molecular analysis of five unrelated Koreans with X-linked CGD. Biochemical analysis demonstrated severe defects in the respiratory burst of these patients except in patient 5. Even though it has been reported that there is no clear correlation between the biochemical or molecular characteristics and clinical manifestations (8), microgranular pattern shown in patient 5 seemed to be related with less severe clinical history. Molecular findings, however, does not seem to be correlated with clinical severity. For example, the same kind of frameshift mutations found in patient 1, 3, and 4 did not lead to the same mortality in all patients.
CGD is a fatal immunodeficiency syndrome characterized by severe recurrent bacterial and fungal infections. These infections occur most commonly in lungs, gastrointestinal tract, skin and the lymph nodes (9). It has been reported that pneumonia occurred at least 80% of the patients and abscesses in more than 50% (2), as was the case in this study subjects. The causative agents in our patients with pneumonia were Burkholderia cepacia (patient 1 and 4), H. influenza (patient 2), S. aureus (patient 2), Mycobaterium spp. (patient 3), which are also known to be commonly isolated. It has been reported that pulmonary colonization of B. cepacia can lead to a rapid and progressive pneumonic illness termed cepacia syndrome in some cases (10). It is noteworthy that B. cepacia was isolated in blood samples of patient 1 and 4 at their death. Liver (Patient 1, 3, and 5), anal (Patient 1), and subcutaneous abscess (Patient 2) occurred in 4 of 5 patients.
In patient 1, a T insertion in exon 13 was found, which led to the formation of a TGA stop codon. This nonsense mutation will result in the synthesis of truncated CYBB gene protein that will be very unstable and easily degraded. This mutation has been previously reported in other CGD patient (3, 11).
The mutation found in patient 2 was a splice-site mutation. Mutations around splice junctions are relatively common (12, 13). But c.1111-1G>T is quite rare. RT-PCR of the case revealed the total absence of exon 11 (sequencing result for the cDNA is not shown here), while his mother have both normal and abnormal bands (Fig. 2).
In patient 3, a G insertion occurred in runs of identical G nucleotides. This kind of mutation is considered to occur because of slipped mispairing at the DNA replication fork, which has been also commonly observed in CYBB (14). The mutation of patient 3 resulted in frameshift, which might have caused severe phenotype as in patient 1 and 4. However, he was 22 yr old and still alive at the time of this study. It seems to be difficult to explain why the same kind of frameshift mutations found in patient 1, 3, and 4 did not lead to the same mortality in all patients.
Missense mutation as shown in patient 5, leading to a single amino acid replacement, is commonly seen in X-CGD. Our case showed p.Leu141Pro mutation. This position is of the N-terminal domain and considered outside of the membrane. Mutations that occur near this site have been reported to show an X91+phenotype, which is defined as normal expression of the CYBB gene protein with no oxidase activity (14). Microgranular pattern of activated NBT, however, may indicate an X91-phenotype (partial loss of protein expression). Further investigation is needed to find the molecular and functional characterization of this case.
Carriers of X-linked CGD are usually detected by the PMA activated NBT test as in our five unrelated families. Carrier detection is very important because sons of a carrier have a 50% risk of inheriting the defect and becoming patients. However, it is not an easy task to make a prenatal diagnosis of a fetus if we rely only on the activated NBT test, because an required amount of phagocytes can not be obtained in the first trimester (15). Molecular genetic study should be done both to confirm the diagnosis and to make prenatal diagnosis easier in the family (16). If the mutation is found in a patient and a carrier, it will be easier to find whether a fetus has the same mutation using genomic DNA sample.
SSCP is an appropriate screening method if mutations occur with no hot spot along the whole gene of which size is too long and has many exons as in CYBB gene. Although there are limitations of finding mutations in long base pairs, SSCP is known to exhibit a high degree of sensitivity in detecting single nucleotide differences (17). Highly conservative sequence of CYBB gene is also favorable aspect for SSCP analysis in this disease.
In summary, we identified mutations of the CYBB gene that are responsible for X-linked CGD in 5 unrelated Korean patients. Three mutations in patients 3, 4 and 5 have not been reported yet. Initial screening by SSCP and selective sequencing was a good strategy for mutation detection in this disease. All the results were quite different from one another, revealing that the molecular defects in Korean patients are also very heterogeneous. Compared with patients of other ethnic group, no specific frequency pattern of mutations was found. Further study is needed to find out whether there are more common mutations among Korean patient population. The results of this study will be a basis for the further study of the molecular and functional features of the NADPH oxidase and helping the families with prenatal diagnosis or genetic counseling.
Figures and Tables
Fig. 1
(A) Compared with control (lane 1, 3, 4), SSCP analysis of patient 1 showed different migration pattern around exon 13 (lane 2), (B) The DNA sequence around exon 13 showed c.1663insT forming a premature TGA stop codon in patient 1.

Fig. 2
(A) The DNA sequence of patient 2 shows a c.1111-1G>T, (B) The cDNA RT-PCR reveals exon II deletion in patient 2 (lane 8). The 694 bp sized fragment included cDNA sequence from 1111 to 1784. Lane 1, 4, 7 were of a control. Lane 2, 5, 8 were of the patient. Lane 3, 6, 9 were of the patient's mother with both normal and abnormal bands in lane 9.
References
1. Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical and clinical features of chronic granulomatous disease. Medicine. 2000. 79:170–200.

2. Winkelstein JA, Marino MC, Johnston RB Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine. 2000. 79:155–169.

4. Meerhof LJ, Roos D. Heterogeneity in chronic granulomatous disease detected with an improved nitroblue tetrazolium slide test. J Leukoc Biol. 1986. 39:699–711.

5. Patino PJ, Perez JE, Lopez JA, Condino-Neto A, Grumach AS, Botero JH, Curnutte JT, Garcia de Olarte D. Molecular analysis of chronic granulomatous disease caused by defects in gp91-phox. Hum Mutat. 1999. 13:29–37.
6. Bannai M, Tokunaga K, Lin L, Kuwata S, Mazda T, Amaki I, Fujisawa K, Juji T. Discrimination of human HLA-DRB1 alleles by PCR-SSCP (single-strand conformation polymorphism) method. Eur J Immunogenet. 1994. 21:1–9.

7. Dinauer MC, Curnutte JT, Rosen H, Orkin SH. A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J Clin Invest. 1989. 84:2012–2016.

8. Gerard B, El Benna J, Alcain F, Gougerot-Pocidalo MA, Grandchamp B, Chollet-Martin S. Characterization of 11 novel mutations in the X-linked chronic granulomatous disease (CYBB gene). Hum Mutat. 2001. 18:163.
9. Curnutte JT. Chronic granulomatous disease: the solving of clinical riddle at the molecular level. Clin Immunol Immunopathol. 1993. 67:S2–S15.
10. Jones AM, Dodd ME, Webb AK. Burkholderia cepacia: current clinical issues, environmental controversies and ethical dilemmas. Eur Respir J. 2001. 17:295–301.

11. Rae J, Newburger PE, Dinauer MC, Noack D, Hopkins PJ, Kuruto R, Curnutte JT. X-linked chronic granulomatous disease: mutations in the CYBB gene encoding the gp91-phox component of respiratory-burst oxidase. Am J Hum Genet. 1998. 62:1320–1331.

12. de Boer M, Bolscher BG, Dinauer MC, Orkin SH, Smith CI, Ahlin A, Weening RS, Roos D. Splice site mutations are a common cause of X-linked chronic granulomatous disease. Blood. 1992. 80:1553–1558.

14. Roos D, de Boer M, Kuribayashi F, Meischl C, Weening RS, Segal AW, Ahlin A, Nemet K, Hossle JP, Bernatowska-Matuszkiewicz E, Middleton-Price H. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996. 87:1663–1681.

15. Skalnik DG, Strauss EC, Orkin SH. CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phox gene promoter. J Biol Chem. 1991. 266:16736–16744.

16. Ariga T, Sakiyama Y, Furuta H, Matsumoto S. Molecular genetic studies of two families with X-linked chronic granulomatous disease: mutation analysis and definitive determination of carrier status in patients' sisters. Eur J Haematol. 1994. 52:99–102.

17. Hayashi K. PCR-SSCP: a simple and sensitive method for detection of mutation in the genomic DNA. PCR Methods Appl. 1991. 1:34–38.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download