Abstract
With the widespread emergence of antimicrobial resistance, combination regimens of ceftriaxone and vancomycin (C+V) or ceftriaxone and rifampin (C+R) are recommended for empirical treatment of pneumococcal meningitis. To evaluate the therapeutic efficacy of meropenem (M), we compared various treatment regimens in arabbit model of meningitis caused by penicillin-resistant Streptococcus pneumoniae (PRSP). Therapeutic efficacy was also evaluated by the final bacterial concentration in the cerebrospinal fluid (CSF) at 24 hr. Each group consisted of six rabbits. C+V cleared the CSF at 10 hr, but regrowth was noted in 3 rabbits at 24 hr. Meropenem monotherapy resulted in sterilization at 10 hr, but regrowth was observed in all 6 rabbits at 24 hr. M+V also resulted in sterilization at 10 hr, but regrowth was observed in 2 rabbits at 24 hr. M+V was superior to the meropenem monotherapy at 24 hr (reduction of 4.8 vs. 1.8 log10 cfu/mL, respectively; p=0.003). The therapeutic efficacy of M+V was comparable to that of C+V (reduction of 4.8 vs. 4.0 log10 cfu/mL, respectively; p=0.054). The meropenem monotherapy may not be a suitable choice for PRSP meningitis, while combination of meropenem and vancomycin could be a possible alternative in the treatment of PRSP meningitis.
Antimicrobial resistance in Streptococcus pneumoniae has become a serious concern throughout the world (1, 2). The prevalence of pneumococcal resistance was reported to be high in some Asian countries, western Europe, South Africa and the southern part of the United States (3-5). According to the increasing prevalence of in vitro pneumococcal resistance, therapeutic options for pneumococcal infections have been changed. Pneumococcus is the most common cause of acute bacterial meningitis in adults, and the mortality rate of pneumococcal meningitis is as high as about 25-30% (6, 7). For pneumococcal meningitis, treatment failure of a single-drug regimen such as penicillin, chloramphenicol, third-generation cephalosporins, and vancomycin has been frequently reported (8-11). The combination regimen of ceftriaxone and vancomycin or ceftriaxone plus rifampin is recommended for empirical treatment of pneumococcal meningitis, especially in countries with a high prevalence of resistance (12-14).
Meropenem, which has an excellent anti-pneumococcal activity and less potential for seizure, could be one of the candidate regimens for pneumococcal meningitis (15). However, there have been only a few studies on the therapeutic efficacy of meropenem or a meropenem-based regimen in the treatment of penicillin-resistant pneumococcal meningitis (16-19). We have herein evaluated the therapeutic efficacy of meropenem monotherapy and the combination of meropenem plus vancomycin for meningitis using a rabbit model of meningitis caused by penicillin-resistant S. pneumoniae (PRSP).
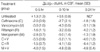
A highly penicillin- and cephalosporin-resistant strain of S. pneumoniae, originally isolated from a 55-yr-old Korean patient with pneumococcal meningitis, was used in this study. The strain was grown overnight on blood agar. The plates were flooded with phosphate-buffered saline and aliquots of the resultant suspension were frozen at -70℃. Aliquots were thawed and diluted to a concentration of 5×107 cfu/mL, and 0.5 mL of this was injected intracisternally into each rabbit. The minimum inhibitory concentrations (MICs) of antibiotics were determined by broth microdilution with Mueller-Hinton broth supplemented with 3% lysed horse blood. The minimum bactericidal concentrations (MBCs) were determined by plating 10-µL aliquots from clear microtiter wells onto blood agar. The MICs and MBCs were as follows: penicillin 2 µg/mL and 4 µg/mL, ceftriaxone 4 µg/mL and 8 µg/mL, vancomycin 0.5 µg/mL and 0.5 µg/mL, rifampin 0.12 µg/mL and 0.25 µg/mL, and meropenem 0.5 µg/mL and 0.5 µg/mL, respectively (Table 1). This strain was intermediately-resistant to meropenem, while it was susceptible to vancomycin (MIC 0.5 µg/mL) and rifampin (MIC 0.12 µg/mL) (20).
Seven treatment groups were evaluated: ceftriaxone (100 mg/kg), vancomycin (20 mg/kg), rifampin (15 mg/kg), meropenem (125 mg/kg), ceftriaxone plus vancomycin, ceftriaxone plus rifampin, and meropenem plus vancomycin. Each antibiotic was given intravenously twice at a 5-hr interval, except ceftriaxone which was given once. Each treatment group consisted of six rabbits. Untreated controls (3 rabbits) received saline alone.
We used the rabbit model originally described by Dacey and Sande (21). The study protocol was approved by the Institutional Animal Care and Use Committee. Young male New Zealand White rabbits weighing 2.5 to 3.0 kg were anesthetized by intramuscular injections of ketamine (40 mg/kg) and xylazine (15 mg/kg) and were immobilized for the induction of meningitis and sampling of cerebrospinal fluid (CSF). An inoculum that contained approximately 107 cfu of multidrug resistant pneumococci was directly injected into the cisterna magna through a spinal needle. Eighteen to twenty hours after the intracisternal injection of the pneumococcal inoculum, CSF was withdrawn and antimicrobial agents were administered through a peripheral ear vein. A second dose of each agent, except for ceftriaxone that was given once, was given five hours later. Untreated and treated animals were euthanized 26 and 40 hr after intracisternal inoculation, respectively.
Bacterial concentrations in CSF were measured 0, 5, 10, and 24 hr after the start of antimicrobial therapy by plating undiluted CSF and serial 10-fold dilutions of CSF (100 µL) on sheep blood agar and incubating in 5% CO2 at 35℃ for 24 hr (21). The bacterial killing rate in the CSF was assessed by a reduction of bacterial concentrations in the CSF during the interval. The final therapeutic efficacy of each treatment group was assessed by the final bacterial concentrations in the CSF and the numbers of animals surviving at 24 hr. In vivo synergism was defined when combination therapy reduced the bacterial concentration from the start of therapy by more than 1 log10 cfu/mL compared with the sum of the reduction with each agent alone (21). Antibiotic concentrations were measured in CSF sampled from treated rabbits at 60 min (peak) and 5 hr (trough) after each antibiotic dose and in serum sampled 30 min and 5 hr after an intravenous dose. All specimens were frozen at -70℃ until analysis. Ceftriaxone and meropenem concentrations were determined by disk diffusion micro-bioassay using Escherichia coli (ATCC 10536), and the vancomycin and rifampin concentrations were determined by disk diffusion micro-bioassay using Bacillus subtilis (ATCC 6633) and Micrococcus lutea (ATCC 9341), respectively.
The mean changes in bacterial concentrations in each treatment group were compared by ANOVA (Newman-Keuls multiple comparison test) and Tukey's honestly significant difference (HSD) test as a post hoc test. Mann-Whitney test was used for comparison of two groups of results. A p value of <0.05 was considered significant.
The peak antibiotic concentrations in the CSF and serum obtained 60 min and 30 min after the initial dose of antibiotics, respectively and the trough concentrations obtained just before the second dose are presented in Table 2. In regard to meropenem a high dose (125 mg/kg) was required to maintain the level of CSF drug concentration compared to the usual dose of meropenem in human, and the peak and the lowest levels of mezopenem were 5.7 and 0.7 µg/mL, respectively. The peak CSF concentration of ceftriaxone was lower than the MBC for the infecting strain, while peak concentrations of vancomycin and rifampin in the CSF were three-fold and two-fold higher than the MBC, respectively.
CSF bacterial concentrations in each rabbit treatment group are shown in Fig. 1, 2, 3, and 4. Meropenem resulted in sterilization at 10 hr but regrowth was observed in all 6 rabbits at 24 hr (Table 3, Fig. 1). Meropenem plus vancomycin resulted in sterilization in all 6 rabbits at 10 hr, and regrowth was observed in 2 rabbits at 24 hr (Table 3 and Fig. 2). Meropenem therapy resulted in 5 log10 cfu/mL reduction in the bacterial concentration within 10 hr, but regrowth was observed at 24 hr (Table 4 and Fig. 1). Meropenem plus vancomycin resulted in 4.8 log10 cfu/mL reduction in the bacterial concentration within 10 hr. The effect persisted until 24 hr (Table 4 and Fig. 2), but regrowth was observed in 2 rabbits (Fig. 2). Compared with meropenem and ceftriaxone, ceftriaxone showed a less reduction of bacteria at 10 hr and at 24 hr (-1.8±1.8 ΔLog10 cfu/mL, mean±SD). Meropenem showed eradication of bacteria at 10 hr and reduction of bacteria at 24 hr (-1.3±1.9 ΔLog10 cfu/mL, mean±SD), (p=0.337 at 24 hr) (Table 4 and Fig. 1). When meropenem or vancomycin monotherapy was compared with the combination of meropenem plus vancomycin (M+V), ANOVA showed a statistically significant difference (p=0.004), and the difference between M+V and meropenem was significant, albeit without synergism (reduction of 4.8 vs. 1.8 log10 cfu/mL, respectively; p=0.003), while the difference between M+V and vancomycin was not (p=0.181) by Tukey's HSD test as a post hoc test (Fig. 2). Compared with ceftriaxone plus vancomycin and meropenem plus vancomycin, the combination of ceftriaxone plus vancomycin showed -4.0±1.2 ΔLog10 cfu/mL in CSF (mean±SD) and meropenem plus vancomycin showed -4.8±0.4 ΔLog10 cfu/mL (mean±SD), (p=0.054) (Table 4, Fig. 3). Both ceftriaxone plus vancomycin and meropenem plus vancomycin showed eradication in all 6 rabbits at 10 hr, however, meropenem plus vancomycin had a tendency to have superior activity against experimental strain at 24 hr (Table 3, Fig. 3). Compared with ceftriaxone plus vancomycin, meropenem plus vancomycin and ceftriaxone plus rifampin, only ceftriaxone plus rifampin showed eradication in all 6 rabbits at 24 hr (Table 3, Fig. 4).
Treatment of pneumococcal meningitis has been complicated by the emergence of in vitro pneumococcal resistance during the past two decades. Ceftriaxone or cefotaxime can be a drug of choice for pneumococcal meningitis by a penicillin non-susceptible strain, if the organism is susceptible to them (13, 14). However, therapeutic failures have been documented when these agents have been used for meningitis caused by ceftriaxone-resistant strains (MIC≥2 µg/mL) (8-10, 22, 23). In our experiment, the ceftriaxone monotherapy was the least effective regimen for the treatment of rabbit meningitis due to PRSP. Given the strong association of ceftriaxone (or cefotaxime) resistance with penicillin resistance among pneumococci, the monotherapy of these agents is not a safe initial choice, especially in countries where penicillin resistance is highly prevalent.
With the emergence of β-lactam antibiotic resistance among pneumococci, vancomycin has assumed an important role in the treatment of meningitis caused by strains that are not susceptible to penicillin and cefotaxime (or ceftriaxone). However, treatment failure of vancomycin monotherapy was also reported (11). The mean peak concentrations of vancomycin in the CSF should be four- to eight-fold higher than the MBC for sterilization of CSF in a rabbit model (24). In our model, however, the mean peak concentration of vancomycin (peak, 1.2 µg/mL, trough, 0.8 µg/mL) was only two-fold higher than the MBC (0.5 µg/mL) of the used strain. As a result, vancomycin failed to sterilize the CSF in some rabbits at 24 hr, although it showed a rapid bactericidal activity initially.
Accumulation of clinical data on treatment failures of single-drug regimens has led to a combination of a 3rd generation cephalosporin and vancomycin or 3rd generation cephalosporin and rifampin as an initial empirical regimen for pneumococcal meningitis (12-14). Although these combination regimens are the standard option for treatment of bacterial meningitis in the countries of high prevalence of PRSP, a new option for monotherapy has been investigated.
One of the new potential options is carbapenem, which shows an excellent in vitro activity against S. pneumoniae. However, the clinical usefulness of imipenem-cilastatin for the treatment of bacterial meningitis may be limited by the increased incidence of drug-related seizure activity (25). On the other hand, meropenem is considerably less likely to cause seizures (26). Meropenem is well tolerated as either a bolus or an infusion, and clinical trials have shown similar incidences of adverse events to those observed with cephalosporin-based treatments. It is well tolerated by the central nerve system (CNS), with infrequent reports of seizures, and can therefore be used at high doses in patients with meningitis (15). Penetration of meropenem into the CSF of patients with inflamed meninges is good at a dose of 40 mg/kg in human (27). In our study, a higher dose (125 mg/kg) of meropenem was administered to maintain the CSF drug level as in the previous studies (18, 19). No significant adverse reactions were noted with this high dose. Despite the adequate concentration of meropenem in the CSF, meropenem monotherapy showed regrowth of bacteria at 24 hr, which suggested that meropenem had a bacteriostatic effect on this strain. Intermediate resistance of meropenem (MIC 0.5 µg/mL, MIC breakpoint; susceptible <0.25 µg/mL, intermediate 0.5 µg/mL, resistant >1 µg/mL) to the strain we used may be the main cause of the failure in bacterial eradication.
Although the in vitro activity of meropenem against S. pneumoniae is excellent in general, recent reports showed an increasing prevalence of isolates with a decreased susceptibility to meropenem, especially among penicillin-resistant strains (28, 29). Given the recent epidemiologic data (28, 29) and similar results by Friedland et al. (18), meropenem monotherapy may not be a good option for the treatment of pneumococcal meningitis caused by resistant strains.
The therapeutic efficacy of the combination of meropenem and vancomycin for the penicillin-resistant pneumococcal meningitis model was recently reported by Gerber et al. who showed no significant advantage over meropenem alone (19). Data from our study showed that the combination of meropenem and vancomycin was superior to meropenem alone. But this combination was not superior to vancomycin alone. These findings are mainly associated with the antibiotic (meropenem and vancomycin) susceptibility of the strain we used. The combination of meropenem and vancomycin was not synergistic under the experimental condition.
In our model with a rifampin-susceptible strain, the combination of ceftriaxone and rifampin showed a much higher bactericidal activity than that of ceftriaxone and vancomycin or meropenem plus vancomycin. Rifampin resistance of S. pneumoniae may be uncommon (30). However, the bactericidal effect of beta-lactam antibiotics or vancomycin was reduced by the addition of rifampin in vitro (31). In addition, the therapeutic efficacy of rifampin-based combination in the treatment of pneumococcal meningitis caused by a rifampin-resistant strain has not been documented. Therefore, rifampin should be used in combination if the organism is susceptible to rifampin or if there is a delay in the treatment response to regimens without rifampin.
The data from this study suggest that meropenem alone is not effective in the treatment of pneumococcal meningitis caused by a meropenem-intermediate strain. Meropenem monotherapy may not be a suitable choice for PRSP meningitis, while the combination of meropenem and vancomycin could be a possible alternative in the treatment of PRSP meningitis. The clinical usefulness of these regimens may depend on the epidemiologic situation of meropenem resistance.
Figures and Tables
Fig. 1
Therapeutic efficacies of ceftriaxone and meropenem by mean bacterial concentrations in the CSF. The difference in ΔLog10 cfu/mL in CSF at 24 hr was not statistically significant.

Fig. 2
Therapeutic efficacies of meropenem, vancomycin, and M+V by mean bacterial concentrations in the CSF. The difference in ΔLog10 cfu/mL in CSF at 24 hr between M+V and meropenem was significant (p=0.003), but M+V and vancomycin was not significant (p=0.181) by Tukey's HSD test.

Fig. 3
Therapeutic efficacies of C+V and M+V by mean bacterial concentrations in the CSF. The difference in ΔLog10 cfu/mL in CSF at 24 hr was not significant (p=0.054).

Fig. 4
Therapeutic efficacies of C+V, M+V, and C+R by mean bacterial concentrations in the CSF. The difference in ΔLog10 cfu/mL in CSF at 24 hr was not significant.

ACKNOWLEDGEMENTS
This work was supported partly by a research grant from the Samsung Biomedical Research Institute and the Samsung Medical Center, and partly by the Asian-Pacific Research Foundation for Infectious Diseases (ARFID), Seoul, Korea. We are indebted to Ms. Joung-Hwa Jin for her technical assistance with the rabbit meningitis model.
References
1. Tan TQ. Antibiotic resistant infections due to Streptococcus pneumoniae: impact on therapeutic options and clinical outcome. Curr Opin Infect Dis. 2003. 16:271–277.

2. Tomasz A. New faces of an old pathogen: emergence and spread of multidrug-resistant Streptococcus pneumoniae. Am J Med. 1999. 107:55S–62S.

3. Song JH, Lee NY, Ichiyama S, Yoshida R, Mirakata Y, Fu W, Chongthaleong A, Aswapokee N, Chiu CH, Lalitha MK, Thomas K, Perera J, Yee TT, Jarnal F, Warsa UC, Vinh BX, Jacobs MR, Appelbaum PC, Pai CH. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Clin Infect Dis. 1999. 28:1206–1211.
4. Forward KR. The epidemiology of penicillin resistance in Streptococcus pneumoniae. Semin Respir Infect. 1999. 14:243–254.
5. Hoban DJ, Doern GV, Fluit AC, Roussel-Delvallez M, Jones RN. Worldwide prevalence of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001. 32:Suppl 2. S81–S93.
6. Auburtin M, Porcher R, Bruneel F, Scanvic A, Trouillet JL, Bedos JP, Regnier B, Wolff M. Pneumococcal meningitis in the intensive care unit: prognostic factors of clinical outcome in a series of 80 cases. Am J Respir Crit Care Med. 2002. 165:713–717.
7. Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, Swartz MN. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993. 328:21–28.
8. Bradley JS, Connor JD. Ceftriaxone failure in meningitis caused by Streptococcus pneumoniae with reduced susceptibility to beta-lactam antibiotics. Pediatr Infect Dis J. 1991. 10:871–873.
9. Sloas MM, Barrett FF, Chesney PJ, English BK, Hill BC, Tenover FC, Leggiadro RJ. Cephalosporin treatment failure in penicillin- and cephalosporin-resistant Streptococcus pneumoniae meningitis. Pediatr Infect Dis J. 1992. 11:662–666.
10. Pacheco TR, Cooper CK, Hardy DJ, Betts RF, Bonnez W. Failure of cefotaxime treatment in an adult with Streptococcus pneumoniae meningitis. Am J Med. 1997. 102:303–305.

11. Viladrich PF, Gudiol F, Linares J, Pallares R, Sabate I, Rufi G, Ariza J. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother. 1991. 35:2467–2472.

13. Kaplan SL, Mason EO Jr. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Rev. 1998. 11:628–644.

15. Hurst M, Lamb HM. Meropenem: a review of its use in patients in intensive care. Drugs. 2000. 59:653–680.
16. Vandecasteele SJ, Verhaegen J, Colaert J, Van Caster A, Devlieger H. Failure of cefotaxime and meropenem to eradicate meningitis caused by an intermediately susceptible Streptococcus pneumoniae strain. Eur J Clin Microbiol Infect Dis. 2001. 20:751–752.

17. John CC, Aouad G, Berman B, Schreiber JR. Successful meropenem treatment of multiply resistant pneumococcal meningitis. Pediatr Infect Dis J. 1997. 16:1009–1011.

18. Friedland IR, Paris M, Ehrett S, Hickey S, Olsen K, McCracken GH Jr. Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1993. 37:1630–1636.

19. Gerber CM, Cottagnoud M, Neftel KA, Tauber MG, Cottagnoud P. Meropenem alone and in combination with vancomycin in experimental meningitis caused by a penicillin-resistant pneumococcal strain. Eur J Clin Microbiol Infect Dis. 1999. 18:866–870.

20. NCCLS. Performance Standards for Antimicrobial Susceptibility Testing. Eleventh Informational Supplement. 2001. 21:M100.
21. Dacey RG, Welsh JE, Scheld WM, Winn HR, Jane JA, Sande MA. Alterations in cerebrospinal fluid outflow resistance in experimental bacterial meningitis. Trans Am Neurol Assoc. 1978. 103:142–146.
22. John CC. Treatment failure with use of a third-generation cephalosporin for penicillin-resistant pneumococcal meningitis: case report and review. Clin Infect Dis. 1994. 18:188–193.

23. Catalan MJ, Fernandez JM, Vazquez A, Varela de Seijas E, Suarez A, Bernaldo de Quiros JC. Failure of cefotaxime in the treatment of meningitis due to relatively resistant Streptococcus pneumoniae. Clin Infect Dis. 1994. 18:766–769.

24. Ahmed A, Jafri H, Lutsar I, McCoig CC, Trujillo M, Wubbel L, Shelton S, McCracken GH Jr. Pharmacodynamics of vancomycin for the treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1999. 43:876–881.

25. Balfour JA, Bryson HM, Brogden RN. Imipenem/cilastatin: an update of its antibacterial activity, pharmacokinetics and therapeutic efficacy in the treatment of serious infections. Drugs. 1996. 51:99–136.
26. Lowe MN, Lamb HM. Meropenem: an updated review of its use in the management of intra-abdominal infections. Drugs. 2000. 60:619–646.
27. Dagan R, Velghe L, Rodda JL, Klugman KP. Penetration of meropenem into the cerebrospinal fluid of patients with inflamed meninges. J Antimicrob Chemother. 1994. 34:175–179.

28. Pikis A, Donkersloot JA, Akram S, Keith JM, Campos J, Rodriguez WJ. Decreased susceptibility to imipenem among penicillin-resistant Streptococcus pneumoniae. J Antimicrob Chemother. 1997. 40:105–108. Erratumin: J Antimicrob Chemo Ther 1997; 40: 462.
29. Fuchs PC, Barry AL, Brown SD. Pneumococcal susceptibility to meropenem. J Antimicrob Chemother. 1996. 37:1036–1037.

30. Doern GV, Brueggemann A, Holley HP Jr, Rauch AM. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996. 40:1208–1213.

31. Doit CP, Bonacorsi SP, Fremaux AJ, Sissia G, Cohen R, Geslin PL, Bingen EH. In vitro killing activities of antibiotics at clinically achievable concentrations in cerebrospinal fluid against penicillin-resistant Streptococcus pneumoniae isolated from children with meningitis. Antimicrob Agents Chemother. 1994. 38:2655–2659.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download