Abstract
Background
Fundamental echocardiography has some drawbacks in patients with difficult-to-image echocardiograms. The aim of this study is to evaluate impact of contrast echocardiography (CE) on ventricular function assessment and clinical diagnosis in routine clinical echocardiography.
Methods
Two hundred sixty patients were prospectively enrolled over 3 years in 12 medical centers in Korea. General image quality, the number of distinguishable segments, ability to assess regional wall motion, left ventricular (LV) apex and right ventricle (RV) visualization, LV ejection fraction, changes in diagnostic or treatment plan were documented after echocardiography with and without ultrasound contrast agent.
Results
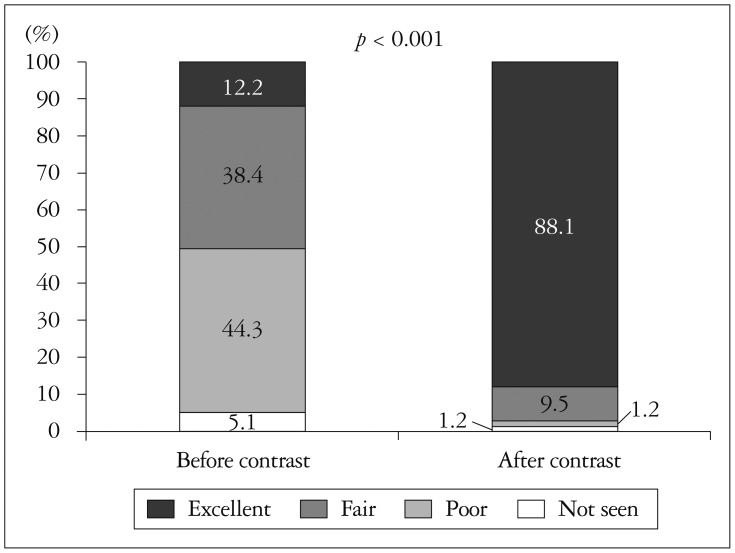
Poor or uninterpretable general image was 31% before contrast use, and decreased to 2% (p<0.05) after contrast use. The average number of visualized LV segments was 9.53 before contrast use, and increased to 14.46 (p<0.001) after contrast use. The percentage of poor or not seen LV regional wall motion was decreased from 28.4% to 3.5% (p<0.001). The percentage of poor or not seen LV apex and RV was decreased from 49.4% to 2.4% (p<0.001), from 30.5% to 10.5% (p<0.001), respectively. Changes in diagnostic procedure and treatment plan after CE were 30% and 29.6%, respectively.
Go to : 
Echocardiography has become an integral diagnostic tool in a clinical field of cardiology.1) Progress of acoustic and digital image processing technology has made echocardiography readily available, convenient, inexpensive, and time-saving diagnostic modality, whereas inherent disadvantages of cardiac ultrasound still exist.2)3)4) Endocardial border delineation (EBD) is important to evaluate left ventricular (LV) volume and ejection fraction (EF). Especially patients with obesity, lung disease, mechanical ventilation, surgical drains and tubes may have a poor echo window.5)6)7)8) LV volume and EF were consistently underestimated by baseline echocardiography than cardiac magnetic resonance image.9)10) Low level of confidence with suboptimal echocardiography leads to repetitive test or other types of diagnostic examination, causing increased patient's medical cost and healthcare system burden.11)12)13) About 15% of routine echocardiogram and up to 30% of echocardiogram in critically ill patients are suboptimal.14) Highly subjective and examiner dependent feature is another pitfalls of echocardiogram without contrast. Inter-observer and intra-observer variability of non-contrast echocardiography (CE) have been under criticism.
CE uses high molecular weight microbubble enclosed by lipids, albumin, or surfactant.15)16) Very low mechanical index (MI) ultrasound has enabled more pronounced nonlinear echo signal from microbubble while suppressing linear echo signal from myocardium.15)16) Clear left ventricular opacification (LVO) and EBD can be acquired by using ultrasound contrast agents (UCAs) in patients with poor image on harmonic echocardiography. CE boasts 95% of EBD and conversion of 75% of previously non-diagnostic, poor echo results to interpretable ones.17) Trainee or novice in echo labs get the benefit more than expert from CE in not-echo-friendly circumstance as intensive care unit (ICU).18)19) Impact of CE on clinical decision making was reported in US20) and impact of CE in ICU patients also reported in Korea.21) We examined impact of CE on assessment of LV function and diagnosis in routine clinical echocardiography in Korea.
Go to : 
From March, 2008 to March, 2011, 260 patients were prospectively enrolled at 12 Korean teaching hospitals. Following routine rest echocardiography, discretionary CE was performed on same patients. The study was approved by Institutional Review Committees at each hospital. A total of 12 expert physicians were involved in care of patients who enrolled. Myocardial CE and stress echocardiography were excluded.
Two-dimensional and spectral Doppler echocardiography were recorded from apical and parasternal windows. Second harmonic imaging and high MI (1.0 to 1.5) were used to acquire echocardiography without contrast. When routine echocardiogram was deemed suboptimal to interpret, Definity (Lantheus Medical Imaging, North Billerica, MA, USA) or SonoVue (Bracco Diagnostics, Milan, Italy) were delivered by bolus injection or by continuous infusion and MI was adjusted to low level (0.3 to 0.5). Both images were stored digitally and interpreted within several hours.
Two independent observers participated. Conventional echocardiography was interpreted by one observer and then CE image was interpreted by the same observer. Another observer evaluated full echocardiographic results with and without contrast at once. Target variables were: global image quality, visualized LV segments, LV apex, LV EF, right ventricle (RV). Semi-quantitative grading was used to categorical variables.
After baseline echocardiography is done, attending physician was contacted and has answered further diagnostic and management plan. CE result was exposed to the same physician, and he or she was questioned about possible changes of diagnostic and treatment plan.
Within 24 h after UCAs injection, any of all following: death, cardiac arrest, myocardial infarction, arrhythmia requiring immediate intervention, anaphylactoid reaction, or hypotension were regarded safety end points.
SPSS for Windows (version 12.0; SSPS Inc., Chicago, IL, USA) was used for statistical analysis. Study population statistics were presented as mean ± SD for continuous variables and count percentages for categorical variables. McNemar test was used to reveal significant proportional differences between two groups. Wilcoxon signed rank test was used to compare the number of visualized LV segments before and after contrast. A p value of 0.05 or lower was considered to be statistically significant.
Go to : 
A total of 260 patients with routine or portable echocardiography who underwent CE were enrolled. Baseline patient characteristics are listed in Table 1. Mean age was 63.2 ± 15.1, male patients were 57.7%, cardiology patients were 66.5%, outpatients were 16.9%, and patients in any kinds of ICU were 32.4%. Table 2 shows baseline echocardiographic characteristics. Routine echocardiography was 80.8% of all test, portable echocardiography was remaining 19.2%. Patients with mechanical ventilator were 13.8%. Echocardiography vendors were Acuson (43.8%), GE (33.5%), and Philips (22.7%). UCAs used in study were mostly Definity (97.3%). Microbubble contrast was bolus injected (61.2%) or continuously infused (38.8%). Heart failure (31.2%) and coronary artery disease (45.8%) were main reasons for echocardiography (Table 3).
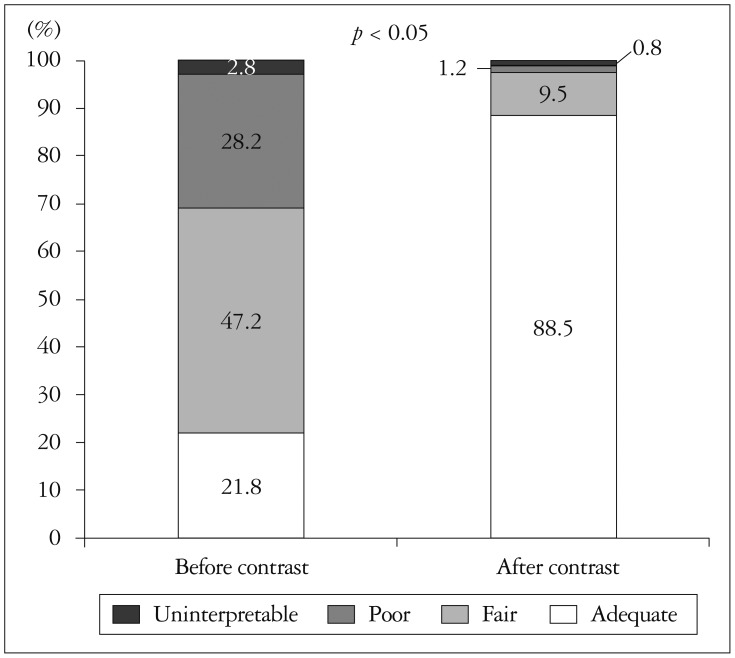
General image quality was classified under four headings; adequate (full endocardial visualization), fair (greater than 50% visualized endocardium), poor (minimal endocardial visualization), or uninterpretable (only epicardium visualized). Before contrast delivery, poor or uninterpretable echocardiography comprised 31% which decreased to 2% after contrast delivery (Fig. 1). The number of visualized LV segments per person was 9.53 before contrast, which increased significantly to 14.46 after contrast (p<0.001). The percentages of inadequate assessment of LV wall motion, before and after contrast, were 28.4% and 3.5%, respectively (p<0.001). LV apex delineation was also greatly increased by contrast. Baseline echocardiography has resulted in 49.4% of unsatisfactory echo views to rule out LV thrombus confidently. CE revealed LV apex more clearly, only 2.4% of all subject was ambiguous (Fig. 2). In patients whose LV EF was calculated, CE made a small but significant increase of EF; from 48.38% to 49.62% (p<0.05). The percentage of poor or not seen RV from apical view were 30.5% without contrast, 10.5% with contrast (p<0.001).
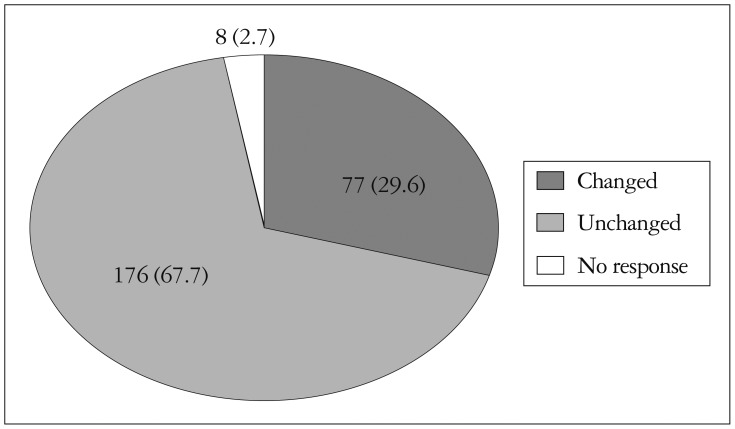
CE influenced clinical diagnostic and drug management plan. Thirty percent of all CE result has made changes of further diagnostic procedure (i.e., cancelling nuclear imaging, transesophageal echocardiography, or diagnostic angiography). Treatment plan was also changed by physicians after CE review, 29.6% of all subjects (Fig. 3).
Among 260 patients, no adverse reaction including back pain, renal pain, chest pain, headache, dizziness, flushing, nausea, vomiting, or others was reported within 24 h after UCAs injection.
Go to : 
This Korean multicenter study showed that CE augmented general image quality, confidence, global and regional LV function assessment, RV image quality and influenced physician's diagnostic and management plan. Suboptimal echo images with routine and portable echocardiography necessitate other expensive medical modalities.2)3)4) CE-LVO produces clearer EBD than fundamental echocardiography, especially in patients with obesity, chronic obstructive lung disease, altered mentality who are difficult to image.1)22)23)24) Commercially available contrasts have prolonged time span of opacification and high blood to myocardium signal ratio.25) UCAs are hemodynamically inert and have a same rheology as blood cells.26)27) In addition, CE is useful in stress echocardiography,28) LV thrombus or mass detection,29) RV and great vessel examination,30)31) mitral regurgitation and aortic stenosis grading.32)33)
Large multicenter prospective clinical trial in US was reported in 2009 by Kurt et al.20) A total of 632 patients with technically difficult images were enrolled. Baseline patient characteristics in US study and in this Korean study are almost same except mean body surface area (BSA). An average BSA in US study was 2.09 m2, whereas an average BSA in Korean study was 1.65 m2. In general image quality, 11.7% of patients were uninterpretable in US study, but 2.8% of patients were uninterpretable in Korean study. This disparity is a possible consequence of higher body mass or BSA in US patients. Echo windows in Korean patients are slightly better than in US patients. CE revealed more endocardial segments assessment than fundamental echocardiography consistently in both studies; 5.3 more visualized segments after contrast in US, 4.9 more visualized segments after contrast in Korea.
Cohen et al.17) reported superiority of CE for LV EBD in 203 patients with suboptimal echo images in a US prospective multicenter trial. Kitzman et al.22) showed improvement of EBD with CE in 211 patients with suspected cardiac disease and suboptimal fundamental echocardiograms in a US multicenter study. In a European multicenter trial, 100 patients planned for routine coronary angiography with or without heart failure were enrolled.34) CE increased inter-observer agreement of regional wall motion. A total of 218 patients with suspected coronary heart disease were enrolled in a European multicenter study by Senior et al.35) CE was safe and effective for LV EBD. Hwang et al.21) reported impact of contrast agent on assessment of LV function and on clinical diagnosis in a Korean prospective multicenter trial on ICU patients. Our study included not only selective population enrolled by previous studies but also routine clinical population. Therefore, this study might reflect well benefit of CE in real clinical practice.
This study has some limitations. First, cost-effectiveness of CE was not fully and specifically calculated with health insurance reimbursement data. Second, the study was not fully blinded by physicians and sonographers. Pre-expectation of observers may cause biased results. Automated assessment function by vendors was not used in EF estimation or wall motion analysis to reduce human errors. Third, readers, observers, and cardiologists in 12 teaching hospitals did not have same exact echocardiographic measurement technique, experience in cardiac echo labs, clinical decision making standards. Lastly, relatively small sample size.
However, this study is a first Korean multicenter study that investigated the utility of CE in a routine clinical echocardiography. And it showed CE was still useful despite Korean patients had relatively low BSA. Also, this study suggested the efficacy of CE in real routine clinical world because study population included not only patients in ICU or with poor echo window but also patients in routine clinical echocardiography environment.
In conclusion, regarding routine clinical echocardiography in Korean population, CE in comparison with fundamental echocardiography improved LV function assessment and clinical decision making.
Go to : 
Acknowledgements
This study was funded by a grant from The Korean Society of Echocardiography.
Go to : 
References
1. Mulvagh SL, DeMaria AN, Feinstein SB, Burns PN, Kaul S, Miller JG, Monaghan M, Porter TR, Shaw LJ, Villanueva FS. Contrast echocardiography: current and future applications. J Am Soc Echocardiogr. 2000; 13:331–342. PMID: 10756254.
2. Lang RM, Mor-Avi V, Zoghbi WA, Senior R, Klein AL, Pearlman AS. The role of contrast enhancement in echocardiographic assessment of left ventricular function. Am J Cardiol. 2002; 90:28J–34J.
3. Senior R, Dwivedi G, Hayat S, Lim TK. Clinical benefits of contrast-enhanced echocardiography during rest and stress examinations. Eur J Echocardiogr. 2005; 6(Suppl 2):S6–S13. PMID: 16360630.
4. Platts DG, Fraser JF. Contrast echocardiography in critical care: echoes of the future? A review of the role of microsphere contrast echocardiography. Crit Care Resusc. 2011; 13:44–55. PMID: 21355829.
5. Hwang JJ, Shyu KG, Chen JJ, Tseng YZ, Kuan P, Lien WP. Usefulness of transesophageal echocardiography in the treatment of critically ill patients. Chest. 1993; 104:861–866. PMID: 8365301.
6. Reilly JP, Tunick PA, Timmermans RJ, Stein B, Rosenzweig BP, Kronzon I. Contrast echocardiography clarifies uninterpretable wall motion in intensive care unit patients. J Am Coll Cardiol. 2000; 35:485–490. PMID: 10676698.
7. Yong Y, Wu D, Fernandes V, Kopelen HA, Shimoni S, Nagueh SF, Callahan JD, Bruns DE, Shaw LJ, Quinones MA, Zoghbi WA. Diagnostic accuracy and cost-effectiveness of contrast echocardiography on evaluation of cardiac function in technically very difficult patients in the intensive care unit. Am J Cardiol. 2002; 89:711–718. PMID: 11897214.
8. Beaulieu Y. Bedside echocardiography in the assessment of the critically ill. Crit Care Med. 2007; 35(5 Suppl):S235–S249. PMID: 17446784.
9. Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Grayburn PA. Administration of an intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: comparison with cine magnetic resonance imaging. J Am Coll Cardiol. 1998; 32:1426–1432. PMID: 9809958.
10. Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Grayburn PA. Effect of contrast enhancement on transthoracic echocardiographic assessment of left ventricular regional wall motion. Am J Cardiol. 1999; 84:1365–1368. PMID: 10614810.
11. Shaw LJ. Impact of contrast echocardiography on diagnostic algorithms: pharmacoeconomic implications. Clin Cardiol. 1997; 20(10 Suppl 1):I39–I48. PMID: 9383601.
12. Thanigaraj S, Nease RF Jr, Schechtman KB, Wade RL, Loslo S, Pérez JE. Use of contrast for image enhancement during stress echocardiography is cost-effective and reduces additional diagnostic testing. Am J Cardiol. 2001; 87:1430–1432. PMID: 11397374.
13. Tardif JC, Dore A, Chan KL, Fagan S, Honos G, Marcotte F, Yu E, Siu S, Dumesnil J, Arsenault M, Koilpillai C, D'onofrio F. Economic impact of contrast stress echocardiography on the diagnosis and initial treatment of patients with suspected coronary artery disease. J Am Soc Echocardiogr. 2002; 15:1335–1345. PMID: 12415226.
14. Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol. 2007; 32:51–96. PMID: 17208647.
15. Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P. Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. Eur J Echocardiogr. 2009; 10:194–212. PMID: 19270054.
16. Porter TR, Abdelmoneim S, Belcik JT, McCulloch ML, Mulvagh SL, Olson JJ, Porcelli C, Tsutsui JM, Wei K. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2014; 27:797–810. PMID: 25085408.
17. Cohen JL, Cheirif J, Segar DS, Gillam LD, Gottdiener JS, Hausnerova E, Bruns DE. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent. Results of a phase III Multicenter Trial. J Am Coll Cardiol. 1998; 32:746–752. PMID: 9741522.
18. Vlassak I, Rubin DN, Odabashian JA, Garcia MJ, King LM, Lin SS, Drinko JK, Morehead AJ, Prior DL, Asher CR, Klein AL, Thomas JD. Contrast and harmonic imaging improves accuracy and efficiency of novice readers for dobutamine stress echocardiography. Echocardiography. 2002; 19:483–488. PMID: 12356343.
19. Makaryus AN, Zubrow ME, Gillam LD, Michelakis N, Phillips L, Ahmed S, Friedman D, Sison C, Kort S, Rosman D, Mangion JR. Contrast echocardiography improves the diagnostic yield of transthoracic studies performed in the intensive care setting by novice sonographers. J Am Soc Echocardiogr. 2005; 18:475–480. PMID: 15891758.
20. Kurt M, Shaikh KA, Peterson L, Kurrelmeyer KM, Shah G, Nagueh SF, Fromm R, Quinones MA, Zoghbi WA. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009; 53:802–810. PMID: 19245974.
21. Hwang HJ, Sohn IS, Kim WS, Hong GR, Choi EY, Rim SJ, Lee SC, Chung WJ, Choi JH, Seo HS, Yoon SJ, Cho KI, Kim HS, Yoon HJ. The cinical impact of bedside contrast echocardiography in intensive care settings: a Korean Multicenter Study. Korean Circ J. 2015; 45:486–491. PMID: 26617651.
22. Kitzman DW, Goldman ME, Gillam LD, Cohen JL, Aurigemma GP, Gottdiener JS. Efficacy and safety of the novel ultrasound contrast agent perflutren (definity) in patients with suboptimal baseline left ventricular echocardiographic images. Am J Cardiol. 2000; 86:669–674. PMID: 10980221.
23. Lafitte S, Dos Santos P, Kerouani A, Robhan T, Roudaut R. Improved reliability for echocardiographic measurement of left ventricular volume using harmonic power imaging mode combined with contrast agent. Am J Cardiol. 2000; 85:1234–1238. PMID: 10802007.
24. Nanda NC, Kitzman DW, Dittrich HC, Hall G. Imagent Clinical Investigators Group. Imagent improves endocardial border delineation, inter-reader agreement, and the accuracy of segmental wall motion assessment. Echocardiography. 2003; 20:151–161. PMID: 12848680.
25. Allen MR, Pellikka PA, Villarraga HR, Klarich KW, Foley DA, Mulvagh SL, Seward JB. Harmonic imaging: echocardiographic enhanced contrast intensity and duration. Int J Card Imaging. 1999; 15:215–220. PMID: 10472523.
26. Keller MW, Segal SS, Kaul S, Duling B. The behavior of sonicated albumin microbubbles within the microcirculation: a basis for their use during myocardial contrast echocardiography. Circ Res. 1989; 65:458–467. PMID: 2752551.
27. Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res. 1994; 74:1157–1165. PMID: 8187282.
28. Dolan MS, Riad K, El-Shafei A, Puri S, Tamirisa K, Bierig M, St Vrain J, McKinney L, Havens E, Habermehl K, Pyatt L, Kern M, Labovitz AJ. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am Heart J. 2001; 142:908–915. PMID: 11685180.
29. Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, DeCara JM, Weinert L, Krausz T, Lang RM. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004; 43:1412–1419. PMID: 15093876.
30. Kimura BJ, Phan JN, Housman LB. Utility of contrast echocardiography in the diagnosis of aortic dissection. J Am Soc Echocardiogr. 1999; 12:155–159. PMID: 9950975.
31. Masugata H, Cotter B, Ohmori K, Kwan OL, Mizushige K, De-Maria AN. Feasibility of right ventricular myocardial opacification by contrast echocardiography and comparison with left ventricular intensity. Am J Cardiol. 1999; 84:1137–1140. PMID: 10569688.
32. Terasawa A, Miyatake K, Nakatani S, Yamagishi M, Matsuda H, Beppu S. Enhancement of Doppler flow signals in the left heart chambers by intravenous injection of sonicated albumin. J Am Coll Cardiol. 1993; 21:737–742. PMID: 8436756.
33. Nakatani S, Imanishi T, Terasawa A, Beppu S, Nagata S, Miyatake K. Clinical application of transpulmonary contrast-enhanced Doppler technique in the assessment of severity of aortic stenosis. J Am Coll Cardiol. 1992; 20:973–978. PMID: 1527309.
34. Hoffmann R, von Bardeleben S, Kasprzak JD, Borges AC, ten Cate F, Firschke C, Lafitte S, Al-Saadi N, Kuntz-Hehner S, Horstick G, Greis C, Engelhardt M, Vanoverschelde JL, Becher H. Analysis of regional left ventricular function by cineventriculography, cardiac magnetic resonance imaging, and unenhanced and contrast-enhanced echocardiography: a multicenter comparison of methods. J Am Coll Cardiol. 2006; 47:121–128. PMID: 16386674.
35. Senior R, Andersson O, Caidahl K, Carlens P, Herregods MC, Jenni R, Kenny A, Melcher A, Svedenhag J, Vanoverschelde JL, Wandt B, Widgren BR, Williams G, Guerret P, la Rosee K, Agati L, Bezante G. Enhanced left ventricular endocardial border delineation with an intravenous injection of SonoVue, a new echocardiographic contrast agent: a European multicenter study. Echocardiography. 2000; 17:705–711. PMID: 11153016.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download