Abstract
Microangiopathic hemolytic anemia occurs in a diverse group of disorders, including thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, and prosthetic cardiac valves. Hemolytic anemia also occurs as a rare complication after mitral valve repair. In this report, we describe a case of microangiopathic hemolytic anemia following myxoma excision and mitral valve repair, which was presented as hemolytic uremic syndrome.
Microangiopathic hemolytic anemia (MAHA) is induced by a mechanical disruption of the red blood cell membrane, which leads to intravascular hemolysis and the appearance of schistocytes, as confirmed by a peripheral blood smear. MAHA occurs in a diverse group of disorders, including thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome (HUS), disseminated intravascular coagulation (DIC) and prosthetic cardiac valves.1) Hemolytic anemia is a rare complication after the mitral valve replacement (MVR), especially in the presence of paravalvular leaks, and even more rarely after mitral valve repair.2)
HUS is characterized by a triad of hemolytic anemia with fragmentation cells, low platelet count and acute renal failure. Bacterial toxins, drugs and systemic disorders are well known causes of HUS.3)
We report a case of MAHA associated with myxoma excision and mitral valve repair, which presented as a case of HUS.
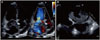
A 71-year-old female was admitted with progressive dyspnea on exertion for 3 days. A transthoracic echocardiogram (TTE) and transesophageal echocardiogram revealed a huge (8.1 × 1.44 cm), highly mobile, echolucent, snake-shaped left atrial (LA) mass with moderate to severe eccentric mitral regurgitation (MR) (Fig. 1).
She underwent surgical removal of LA mass which was 7.0 × 3.3 cm of cardiac myxoma with degenerative change and mitral valve repair using a 26 mm Cosgrove-Edwards ring (Edwards Lifesciences LLC, NY, USA) annuloplasty band with new chorda formation for the A 1, 2 leaflets.
At 18 days after surgery, a complete blood count revealed white blood cell of 9130 103/uL, hemoglobin of 9.7 g/dL, and platelet of 188 103/µL, and reticount was 2.96%. A blood smear exam showed mild normocytic normochromic anemia with anisocytosis and polychromasia. A blood biochemical analysis revealed serum lactate dehydrogenase (LDH) of more than 1800 IU/L, total bilirubin of 1.46 mg/dL (indirect bilirubin, 0.7 mg/dL), aspartate transaminase of more than 2800 IU/L, alanine transaminase of 1471 IU/L, blood urea nitrogen of 34.5 mg/dL, and creatinine of 1.36 mg/dL. There were no specific findings on ultrasonography of kidney and several laboratory tests for autoimmune disease which were conducted to exclude other causes of acute kidney injury. A direct Coombs test was negative and serum haptoglobulin level was 2 mg/dL.
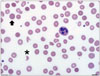
She had complained about dark urine and decreased urine output of 1 L/day on the 34 days after surgery. The vital sign were 130/70 mm Hg of blood pressure, 74/minute of heart rate, 36.3℃ of body temperature, and 20/minute of respiratory rate. Her urine exam was positive for microscopic red blood cell [3–5/high power field (HPF)]. Hemoglobin was 6.8 g/dL, platelet count was 88 103/µL, and creatinine was 1.99 mg/dL. There was no evidence of infection; the patient didn't complain fever and chilling, her C-reactive protein was 0. Also there was no evidence of DIC and she underwent esophagogastroduodenoscopy and colonoscopy to rule out gastrointestinal bleeding, but there was no evidence of bleeding. A blood smear exam (Fig. 2) showed red blood cell fragmentation with a few schistocytes (6/HPF) and polychromasia. There were no specific changes of LDH and haptoglobin levels between the 34 days and 18 days after surgery. A follow-up TTE showed no significant interval change.
Associated with outstandingly elevated LDH level and the presence of circulating schistocytes, we considered MAHA which is a non-immune hemolytic anemia resulting from intravascular red blood cell fragmentation. Because the causes of intravascular hemolysis couldn't be distinguished only by laboratory finding and there was no interval change on follow-up TTE, we preferentially assumed red cell fragmentation syndrome which includes TTP-HUS.
With the overall impression of TTP-HUS, we couldn't wait for the result of ADAMTS13 level which has diagnostic value in acute TTP and it takes time about 1 month only available in one center of Korea, we start plasma exchange immediately and maintained this for 3 weeks. However, her anemia with schistocytosis, hyper-indirect bilirubinemia, and high LDH level were not controlled. During the plasma exchange, the creatinine showed a decreasing number of aspects but never decreased to the normal value and the peak level of creatinine was 1.99 mg/dL. The patient didn't undergo dialysis.
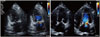
At 47 days after surgery, the TTE showed moderate eccentric MR with multiple jet striking the annuloplasty ring (Fig. 3).
The persistent anemia with schistocytosis and the secondary signs of hemolysis prompted a redo of, the MVR with a tissue valve (27 mm; St. Jude Medical, MN, USA) at 57 days after the initial surgery. The operative finding was an irregular and unclean coaptation surface margin of the mitral valve and but the mitral valve annulus was not dilated.
The anemia with schistocytosis and the secondary signs of hemolysis were immediately resolved after the repeat MVR. The creatinine levels were normalized 4 days after MVR. The patient is doing well, with no abnormal findings and has been followed up in outpatient clinic for 5 months.
To our knowledge, this is the first case report of MAHA presenting as HUS after myxoma excision and mitral valve repair and the first to be resolved after a repeat MVR. Even hemolysis after mitral valve repair is rare, this can be a fatal complication4) and accounts for up to 19% of reoperations.5)
The onset of hemolysis related to mitral valve repair is variable. Lam et al.6) reported that hemolysis generally presented within the first year after operation, while Abourjaili et al.7) reported after a median of 60 days following mitral valve repair.
The severity of the regurgitation varied. One study showed that 3.5% of patients had postoperative hemolysis after mitral annuloplasty; MR was mild in two, moderate in three, and severe in two.8) In contrast, Cerfolio et al.9) reported that two of 10 patients had mild, five had moderate, and only three had severe MR.
Hemolysis is usually suspected in the presence of a falling hematocrit level and recurrent regurgitation.7) Cerfolio et al.9) reported a diagnosis of hemolysis made by decreased serum haptoglobin, elevation of serum LDH, and schistocytosis.
Several previous studies showed the mechanisms of hemolysis as fragmentation of the regurgitant jet by a dehisced annuloplasty ring, rapid acceleration of a jet through a small pararing channel and collision of the regurgitant jet into the prosthetic ring. These mechanisms were associated with high shear stress and could therefore produce hemolysis.6)10)11)
Conflicting results have been presented regarding the prognosis of hemolysis related to mitral valve repair when left untreated. Fix et al.12) found no increase in long-term mortality, while Sheikh et al.13) reported an increase in postoperative morbidity and mortality in patients with mild to moderate residual regurgitation.
Treatments of hemolytic anemia after mitral valve repair were either medical or surgical. Several studies report the use of beta-blockers, pentoxifylline, diuretics, and iron supplements to relieve anemia; however, these only relieved symptoms temporarily.14)15) Abourjaili et al.7) reported seven patients who were treated medically, however four of seven required reoperation due to persistent symptomatic anemia, which resolved after the subsequent surgery. No prospective study has yet identified the ideal treatment, but surgery with either repair or replacement is the desirable choice for treating hemolysis after mitral valve repair. Suri et al.5) reported an association between, mitral valve re-repair and better survival (mortality hazard ratio 0.49, p = 0.05) and improved ejection fraction (55% vs. 44%, p < 0.001) when compared to MVR.
Our case experienced MAHA associated with outstandingly elevated LDH level and the presence of schistocytes occurring 3 weeks after mitral valve repair and was suspected as HUS because other etiology of acute kidney injury were excluded. The failure of plasma exchange for 3 weeks led us to we consider other causes of MAHA. The echocardiogram showed a moderate eccentric MR with multi jets striking the annuloplasty ring, which could cause severe hemolytic anemia. The MAHA with schistocytosis and the secondary signs of hemolysis were completely resolved after the repeat MVR.
In conclusion, visualization of the dynamic flow patterns with echocardiography is important, as is searching for all causes of MAHA as soon as possible in patients with MAHA after mitral valve repair.
Figures and Tables
Fig. 1
A transthoracic echocardiogram (A) and transesophageal echocardiogram (B) showed a huge (8.1 × 1.44 cm), highly mobile, echolucent, snake-shaped left atrial mass with moderate to severe eccentric mitral regurgitation.
References
1. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004; 69:2599–2606.
2. Demirsoy E, Yilmaz O, Sirin G, Baran T, Tekin S, Sener D, Sonmez B. Hemolysis after mitral valve repair: a report of five cases and literature review. J Heart Valve Dis. 2008; 17:24–30.
3. Salvadori M, Bertoni E. Update on hemolytic uremic syndrome: diagnostic and therapeutic recommendations. World J Nephrol. 2013; 2:56–76.
4. Mok P, Lieberman EH, Lilly LS, Schafer AI, DiSesa VJ, Rutherford CR. Severe hemolytic anemia following mitral valve repair. Am Heart J. 1989; 117:1171–1173.
5. Suri RM, Schaff HV, Dearani JA, Sundt TM 3rd, Daly RC, Mullany CJ, Enriquez-Sarano M, Orszulak TA. Recurrent mitral regurgitation after repair: should the mitral valve be re-repaired? J Thorac Cardiovasc Surg. 2006; 132:1390–1397.
6. Lam BK, Cosgrove DM, Bhudia SK, Gillinov AM. Hemolysis after mitral valve repair: mechanisms and treatment. Ann Thorac Surg. 2004; 77:191–195.
7. Abourjaili G, Torbey E, Alsaghir T, Olkovski Y, Costantino T. Hemolytic anemia following mitral valve repair: a case presentation and literature review. Exp Clin Cardiol. 2012; 17:248–250.
8. Ishibashi N, Kasegawa H, Koyanagi T, Ida T. Mechanism of hemolysis after mitral valve repair and new surgical management: prosthetic annuloplasty ring covered with autologous pericardium. J Heart Valve Dis. 2005; 14:588–591.
9. Cerfolio RJ, Orszulak TA, Daly RC, Schaff HV. Reoperation for hemolytic, anaemia complicating mitral valve repair. Eur J Cardiothorac Surg. 1997; 11:479–484.
10. Garcia MJ, Vandervoort P, Stewart WJ, Lytle BW, Cosgrove DM 3rd, Thomas JD, Griffin BP. Mechanisms of hemolysis with mitral prosthetic regurgitation. Study using transesophageal echocardiography and fluid dynamic simulation. J Am Coll Cardiol. 1996; 27:399–406.
11. Yeo TC, Freeman WK, Schaff HV, Orszulak TA. Mechanisms of hemolysis after mitral valve repair: assessment by serial echocardiography. J Am Coll Cardiol. 1998; 32:717–723.
12. Fix J, Isada L, Cosgrove D, Miller DP, Savage R, Blum J, Stewart W. Do patients with less than ‘echo-perfect’ results from mitral valve repair by intraoperative echocardiography have a different outcome? Circulation. 1993; 88(5 Pt 2):II39–II48.
13. Sheikh KH, de Bruijn NP, Rankin JS, Clements FM, Stanley T, Wolfe WG, Kisslo J. The utility of transesophageal echocardiography and Doppler color flow imaging in patients undergoing cardiac valve surgery. J Am Coll Cardiol. 1990; 15:363–372.
14. Maraj R, Jacobs LE, Ioli A, Kotler MN. Evaluation of hemolysis in patients with prosthetic heart valves. Clin Cardiol. 1998; 21:387–392.
15. Koç F, Bekar L, Kadı H, Ceyhan K. Hemolysis and infective endocarditis in a mitral prosthetic valve. Turk Kardiyol Dern Ars. 2010; 38:429–431.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download